Acids and Bases: the Arrhenius concept
The uses of acids and bases were known since time immemorial, although a concise definition was not given until 1884 when Svante Arrhenius proposed his theory of acids and bases. According to Arrhenius,
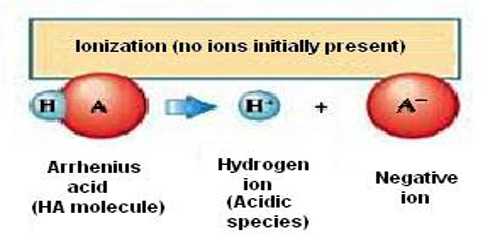
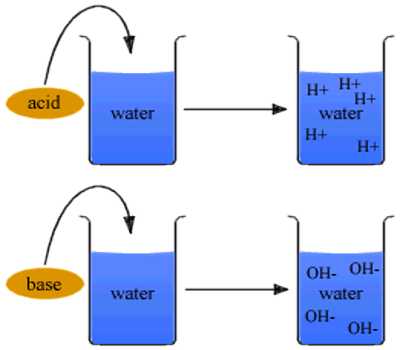
An acid is a compound that yields hydrogen ion (H+) in aqueous solution and a base is a compound that gives hydroxyl (OH–) ions in aqueous solution.
As defined by Arrhenius, acid-base reactions are characterized by acids, which dissociate in aqueous solution to form hydrogen ions (H+) and bases, which form hydroxide (OH−) ions.
Neutralization of a base by an acid was considered essentially to be the reaction:
H+ (aq) + OH– (aq) ↔ H2O (l) … … … (1)
in which the hydrogen and hydroxyl ions combine to form water. Arrhenius definition of acids and bases proved to be inadequate for several reasons.
(i) It was recognized that acid-base reactions took place in solvents other than water.
(ii) Compounds which do not contain hydrogen could release H+ ion by reacting with water:
SO3 (g) + H2O (l) ↔ HH2SO4 (aq) ↔ H+ (aq) + HSO4 (aq)
(iii) Some bases do not contain OH– ions. For example: NH3CaO etc.
It was also believed that a H+ ion cannot exist in free state in any solvent because of its high charge density. A hydrogen ion would combine with one or more molecules of the solvent. In the case of water as solvent H+ ion would combine with water molecules to form what is usually known as a hydronium, hydroxonium, or oxonium ion (H3O+).
H+ + H2O (l) ↔ H3O+ (aq)
These facts together with the studies on catalysis by acids and bases suggested that the ideas of Arrhenius were inadequate and had to be modified.