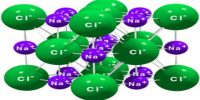
Bonding in Network Covalent Crystals and their Characteristic
Depending on the forces that hold the atoms, molecules or ions together in crystal lattice crystals are classified into four main types.
These are: (i) Ionic crystal, (ii) Molecular crystal, (iii) Network covalent crystal and (iv) Metallic crystal. Here briefly focus on Network covalent crystal.
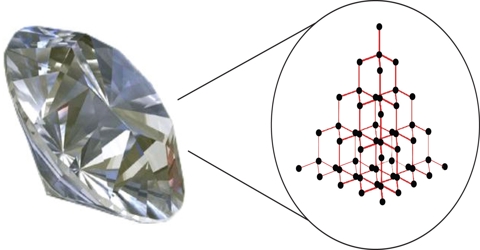
Network covalent crystals are made up of atoms held together by covalent bonds arranged in an extensive three dimensional network. Such crystals are strong and hard. They have very high melting points. A good example is the diamond structure (Figure). In diamond each carbon atom of the lattice is tetrahedrally bonded to four neighbors. Germanium and silicon have diamond like crystal structure. Similar structure is assumed by compounds such as ZnS (Zinc Blende) SiC etc.
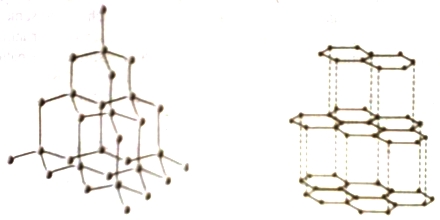
Figure: Structure of diamond; Figure: Structure of graphite
In crystals having diamond like lattice the covalent bonds are arranged in three dimensions. Graphite is an example of the class of crystals which have a two dimensional array of covalent bonds. As shown in Figure-2, graphite has layers of hexagonal networks as in the aromatic series of compounds. In each layer the carbon atoms are held by strong covalent forces but the forces between lavers are weaker. This is why graphite is an excellent lubricant as one laver can slip over another.