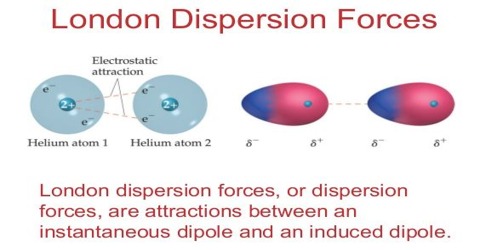
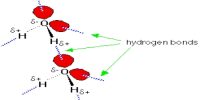
Dispersion forces depend on two aspects of molecular (or atomic) structure.
First, they increase in magnitude with the size and number of electrons and protons in the interacting particles and hence with their molecular masses (or atomic masses). Examples are:
- the boiling points of the noble gases increase from helium to xenon;
- fluorine and chlorine are gases at room temperature and 1 atm pressure, bromine is a liquid, and iodine is a solid;
- large polymers which are electrically neutral are solids with low transition temperatures from solid to liquid. Only dispersion forces are operative in these substances.
Second, dispersion forces depend upon molecular shape via the surface area over which two molecules can be in contact. When the molecules are close to each other lying side by side there an more sites of interaction; the molecules are able to come in contact over a large portion of their length. At each region of contact of the surface of a molecule distortion of the electron distribution may take place and temporary dipoles may be created which will lead to dispersion forces in each region. The larger the surface area of contact the stronger the dispersion forces. Examples are: large polymers with linear structure which are neutral solids have low transition temperatures from solid to liquid.
If the molecule is not linear but has branched chains the number of contact points between the molecules is less and the strength of dispersion forces is also less. For example, n- pentane and 2,2-dimethylpropane have the same molecular mass but the boiling point of n-pentane is higher by 270C than that of the other isomer.
It is important to realize that dispersion forces operate between all molecules, whether or not other forces also operate. Molecules of chloroform, CHC13 are attracted by a combination of dipole-dipole and dispersion forces. Diversion forces are generally weaker than dipole-dipole forces, having valises in the range 0.1 to 5.0 kj mol-1.