The First Law of Thermodynamics
The first law of thermodynamics is also known as the law of conservation of energy. The first law may be stated in different forms:
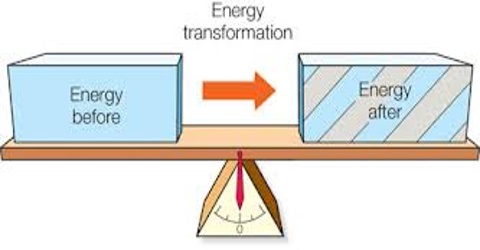
(1) Energy can neither be created nor destroyed: it can only be convened from one form into another.
(2) The total energy of an isolated system is constant.
(3) It is impossible to construct a perpetual motion machine which will create energy out of nothing.
(4) The sum of all of the energy chances for all systems participating in a process must be zero.
(5) If work is produced during a cyclic process, an equivalent amount of heat must also be consumed.
This law has been accepted valid after a long period of skepticism about its inviolability. Numerous attempts were made by scientists to construct a machine by means of which energy could be creased. All such attempts to design “perpetual motion machine” have failed. This is indirect experimental evidence for the first law of thermodynamics.
It is possible to convert matter into energy, as in a nuclear reaction, and under some circumstances to reverse this process. The law of conservation of matter by itself, therefore, does not hold. One may thus consider matter as a form of energy and the law of conservation of energy and the law of conservation of matter are essentially the same. Such transformations of matter into energy then fall under the first law of thermodynamics.
A corollary of the first law is that the internal energy of a system is a function of the state of the system and is independent of the path by which the state has been reached.
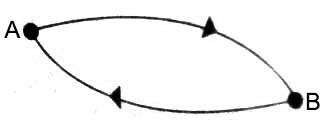
Consider that a system can exist either in state A or in state B (Figure). The internal energy at A is UA and that to B as UB. When the system goes from state A to state B the change in the internal energy is UB – UA. If now the system is brought back to state A by a different path the change in the internal energy will be UA – UB.
According to the first law UB – UA; must he equal to UA – UB in magnitude. If it were not so, creation of energy would be possible, which is against experience. It follows, therefore, that ∆U = UB – UA depends on the internal energies UB and UA at state B and state A, and not on how the system passed from state A to B. The internal energy is said to be a property of state, or a state function. Consequently, in a cyclic process there is no change in internal energy. Or mathematically,
- dU = 0
A cyclic process is one in which a system returns exactly to its original state after undergoing different changes. The symbol § indicates integration over the whole cycle.