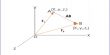
Giant Metal structures, Metal atoms are arranged in layers. The atoms are as close together as possible – a “close-packed” crystal structure. This close-packing explains the high density of metals.
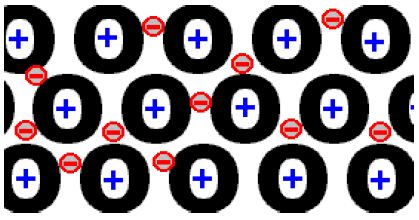
The structure of metals consists of ions surrounded by a ‘sea of electrons’. The outer electrons (negatively charged) from the original metal atoms are free to move around between the positive metal ions (+).
Properties of Metals
There is a strong attractive force between all the delocalised electrons and all the positively charged metal ions. Pulling the metal apart requires breaking these strong bonds, and takes a large amount of energy. Thus metals have high melting and boiling points.
If a strong force is applied to the metal (see diagram below), one layer of atoms can slide over another, and then settle into their dose packed positions again.
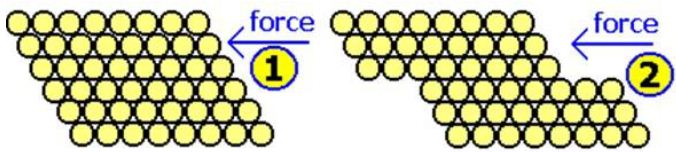
Because the attractive forces are the same in all these positions, the metal stays bonded together. This makes the metal: malleable (can be hammered into different shapes) and ductile (can be pulled out into wires).
If some atoms of a different metal are introduced, then the regular arrangement of the atoms is disrupted, as seen in diagram below. The different size of the new atoms means that the layers cannot slide as easily over each other.
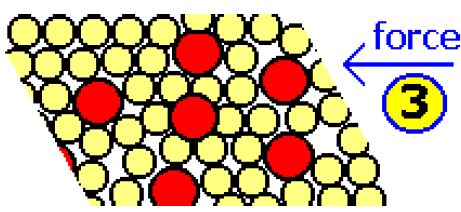
The distortion of the regular dose packed structure by differently sized atoms explains the fact that alloys, such as steel, are harder, and much less malleable than pure metals such as iron.