A homogeneous mixture is a type of mixture that is considered to be the same throughout. We can prepare sorbet by adding some sugar to some water in a glass or jug and stirring the water with a spoon. Again to make hot puffed rice mixture, we can mix the puffed rice with chick-pea, chopped onion, green chili, tomato, etc in a bowl. These mixtures are mixed evenly. Therefore, each part of the mixture seems to be the same. In making sugar juice and hot puffed rice mixture, more than one item is mixed together. So, whatever we prepare by mixing more than one different substance or item is called a mixture.
Liquid examples include pure water, white vinegar, sugar water, corn oil, and blood plasma. Amazingly, homogeneous mixtures are not limited to liquids, they can also be gases and solids. For example, air can be speculated as a homogeneous gas mixture while homogeneous solid mixtures can include alloys such as steel, bronze, brass, and other mixtures such as mineral ores. Examples of homogeneous mixtures are – air in a balloon, salt water in a bottle, or brass in an ingot. Shampoo, hand soap, saltwater, and apple juice are all examples of homogeneous mixtures.
In liquid form, homogeneous mixtures are generally called solutions. A solution is a homogeneous mixture in which one or more substances (the solutes) are dissolved into another substance (the solvent). They consist of a single-phase, be it liquid, gas, or solid, no matter where you sample them or how closely you examine them. The chemical composition is the same for any sample of the mixture.
Task: To know about the solution and homogeneous mixture.
Required accessories: 1 glass (made of glass), 1 spoon, some sugar, and water.
Procedure: Wash the glass clean. Take drinking water in a three-fourth portion of the glass and add one spoon of sugar to it and stir the water with the spoon.
Now pour the solution into a number of pots. Take one spoon from each pot taste can you see the sugar separately?
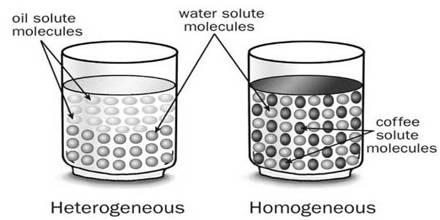
Analysis: No, you do not see any sugar, because, the sugar has been dissolved in the water. Is the sweetness of the mixture tasted from each cupping the same? Yes, the mixture in each of the cups has the same sweetness because the particles of sugar are equally and uniformly distributed everywhere in the mixture.
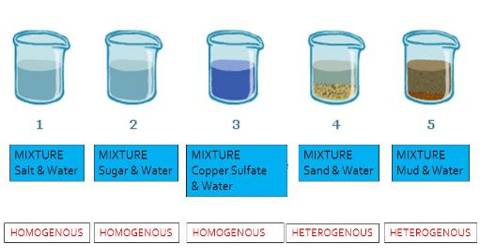
The mixtures whose components are distributed evenly and whose components cannot be easily separated from one another arc called solutions or homogeneous mixtures. That is, solutions are a special type of mixture. Now, you mix salt glucose, fruit juice with water and see whether the obtained mixtures arc homogeneous mixtures or solutions.
Homogeneous Mixture Properties –
- Homogeneous mixtures, more usually called solutions, have a similar uniform appearance and composition throughout.
- Solutions consist of particles as small as atoms or molecules; in other words, too small to be visible to the eye.
- It’s impossible to pick out components of a homogeneous mixture. For example, a sugar solution and white vinegar are homogeneous because only colorless liquids can be seen.
- Homogeneous mixtures only have one phase (state of matter): gas, liquid, or solid. This means you will never observe both a gas and a liquid or a liquid and a solid in a homogeneous mixture.