Ammonia (NH3) is the main composite of nitrogen (N) and hydrogen (H2). It is produced by the usual decomposition of animal and vegetable bodies. The death and decompose of plants and animals cause the nitrogen compounds present in them to get decomposed, giving ammonia.
Ammonia gas is generated and conducted directly from the flask without the introduction of a drying tube into an empty gas washing-bottle which serves to collect any solid particles automatically carried over.
Lab preparation of Ammonia (NH3)
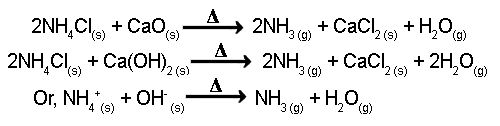
Theory: In the laboratory, a mixture of Ca(OH)2 & NH4Cl is heated in order to produce NH3 gas. In this reaction, CaCl2 & water vapor is also produced.
Description of the process:
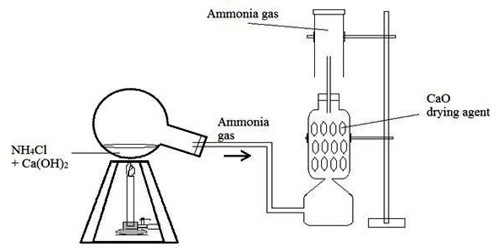
Ammonia gas is generally prepared in the laboratory by quietly heating ammonium chloride (NH4Cl) and slaked lime [Ca(OH)2]. A certain mass of NH4Cl & double of its mass of Ca(OH)2 is taken in a boiling tube. A delivery tube is fitted to the mouth of the boiling tube using a cork, and the boiling tube is clamped to a stand.
The open end of the delivery tube is fitted to the lower end of a tower containing dry CaO, which is being used here as a drying agent. Finally, the side tube at the upper part of the tower is inserted into a dry & inverted gas jar.
Now, if the mixture in the boiling tube is heated slowly, wet NH3 is produced.
Ammonia gas is lighter than air, necessitating its assortment by the downward displacement of air. Because it is highly soluble in water it cannot be collected over it. Ammonia naturally melts at room temperature at a pressure of around 8-10 atmospheres.
The NH3 evolved might be detected –
- By its typical smell.
- By turning humid litmus paper blue.
- By forming dense white clouds of NH4Cl with the stopper from a bottle of HCl.
- By forming a yellow–orange–brown precipitate with Nessler’s solution.
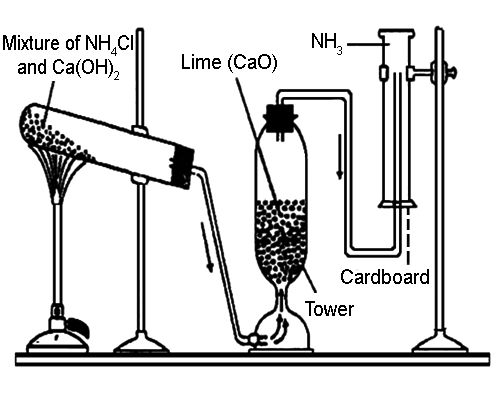
Fig: Preparation of Ammonia in Laboratory.
[Potassium Hydroxide (KOH) or sodium hydroxide (NaOH) can also be used as an alternative of dry slaked lime [(Ca(OH)2]. Since slacked lime is much cheaper than NaOH or KOH, the former is constantly preferred.]
Drying & collection of wet gas: Water vapor is mixed with the prepared NH3. If tips wet gas is passed through the tower containing CaO (drying agent), the water vapor is absorbed by the CaO & dry NH3 gas is obtained. Since NH3 gas is lighter than air, k can be collected in a dry gas jar by downward displacement of air.
It is dried with CaO (quick lime) because CaO and NH3 both are basic compounds so they do not react with each other.
CaO + NH3 —> No reaction
CaO + H2O —> Ca(OH)2
In industries: Most of the ammonia is pretend unnaturally from H2 and N2 by the Haber–Bosch procedure. About 40 billion pounds of ammonia are manufactured yearly in America, regularly by the Haber procedure. The liquid ammonia can be useful directly to the soil as manure. Agricultural use is the main single purpose of manufactured NH3.
N2 + 3H3 ↔ 2 NH3 + Heat.