Xenon forms three binary Fluorides XeF2, XeF4, and XeF6 by the direct union of elements under appropriate experimental conditions.
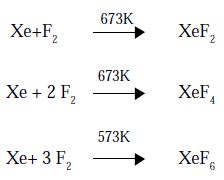
PROPERTIES: Xe F2, Xe F4 and Xe F6 are colourless crystalline solids subliming readily at 298K. They are powerful fluorinating agents. They are readily hydrolysed by even traces of water. For example;
2Xe F2 + 2 H2O → 2Xe + 4HF + O2
Structure: The structure of the three xenon fluorides can be deduced from VSEPR theory. XeF2 and XeF4 have the linear and square planar structure respectively. XeF6, has 7 electron pairs (6 bonding and one lone pair) and thus have a distorted octahedral structure in the gas phase.












