Uses of buffer solutions
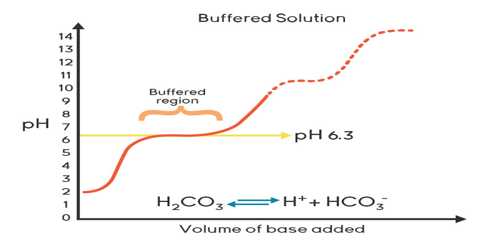
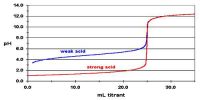
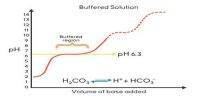
The buffer solution is defined as a solution that resists change in pH upon addition of small quantities of strong acids or strong bases to it.
Strict control of pH is necessary for many biological systems. For example, blood pH is maintained at about 7.4 by a mixture of H2CO3 and HCO3– and protein – phosphate buffers. A change of more than 0.1 unit of pH of blood brings about serious pathological problems. Other body fluids, such as tears, have to be maintained at specific values of pH for normal functioning. Buffering action is necessary to do the job. In many analytical procedures, pH is maintained at the optimum value with help of buffers. Buffers have applications in many industrial processes, particularly in pharmaceutical industries.
There are two types of buffer solutions:
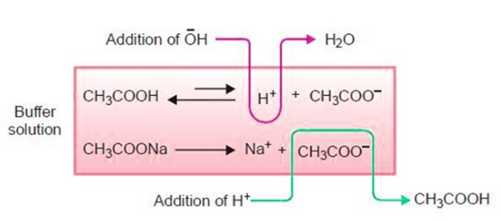
(i) A weak acid and its salt with a strong base. For example, a mixture of CH3COOH and CH3COONa acts as a butter. In this case, CH3COONa is a salt of ethanoic acid with NaOH which is a strong base. This is an acid buffer.
(ii) A weak base and its salt with a strong acid. For example, a mixture of NH4OH and NH4Cl acts as a buffer. In this case, NH4OH is a weak base and NH4Cl is its salt with HCl which is a strong acid. This is a basic buffer.