Any molecule’s solubility in things depends a lot on whether it is a polar molecule or a non-polar molecule. So, polar molecules are soluble in other polar molecules and non-polar molecules are soluble in other non-polar molecules.
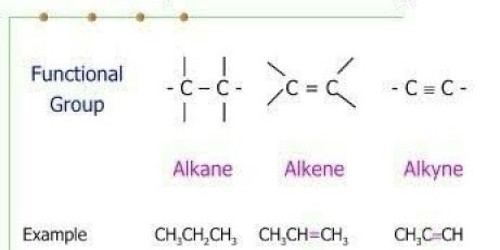
The solubility of Alkanes, Alkenes, and Alkynes:
Solubility of Alkanes
- Alkanes are insoluble in water because they are non-polar.
- They are soluble in various organic solvents. Example: Benzene. ether etc.
- The solubility of alkanes show a gradual decrease with increase in the molecular mass (chain lengths)
Solubility of Alkene
- Alkenes are Insoluble in water because they are also non-polar and covalent compounds.
- Alkenes are Soluble in a various organic solvent. Example: Benzene, ether, alcohol, etc.
Alkanes and alkenes are non-polar molecules. Water, on the other hand, is a polar molecule. So, alkanes and alkenes are not soluble in water.
The solubility of Alkynes
Alkynes are generally nonpolar molecules with little solubility in polar solvents, such as water.
- Insoluble in water.
- Soluble in various organic solvent.
- They are insoluble in water but readily dissolve in organic solvents such as ether, carbon tetrachloride, and benzene.