During chemical reactions, elements do not lose their identity. During nuclear reactions, the nuclei of atoms undergo change and therefore new elements are formed as a result of such reactions. One of the main differences between nuclear reaction and chemical reaction is related to how the reaction takes place in the atom. While nuclear reaction takes place in the atom’s nucleus, the electrons in the atom are responsible for Chemical reactions.
Difference between chemical reactions and nuclear reactions
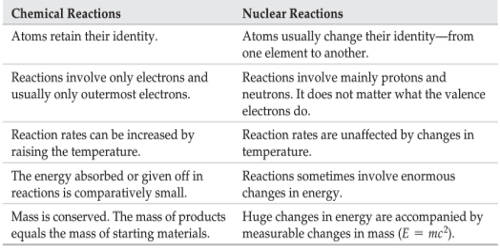
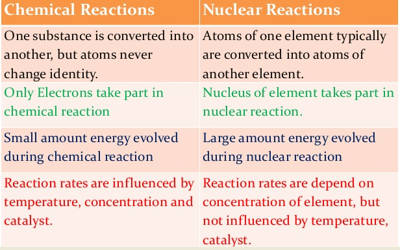
In ordinary chemical reactions, the nuclei of the atoms taking part in a chemical reaction remain unaffected. Only the electrons in the extranuclear part of atoms take part in the chemical process.
Chemical reactions
- These reactions involve some loss, gain or overlap of outer orbital electrons of the reactant atoms. In these reactions, only the electrons in the outermost shell of atoms participate whereas the nuclei of atoms remain unchanged. They involve only a rearrangement of electrons and do not involve changes in the nuclei.
- A chemical reaction is balanced in terms of mass only. It involves a change in an atom’s nucleus, usually producing a different element. The order of energy evolved during a nuclear reaction is much higher than that of a chemical reaction.
- Reactivity of an element towards chemical reactions depends upon the oxidation state of the element.
- The energy changes in any chemical reaction are very much less when compared with nuclear reaction. These reactions are accompanied by relatively small energy changes.
- In chemical reactions, the energy is expressed in terms of kilojoules per mole. Energy changes accompanying nuclear reactions are much larger.
- No new element is produced since the nucleus is unaffected. Different isotopes of an element have nearly the same chemical reactivity.
- The rate of a chemical reaction is largely affected by temperature and pressure.
- The chemical reactions involve the transfer, loss, gain, and sharing of electrons and nothing takes place in the nucleus.
Nuclear reactions
- Nuclear reactions involve the emission of alpha, beta and gamma particles from the nucleus. They involve a change in an atom’s nucleus, usually producing a different element, along with the emission of radiations like α, β, and γ, etc. rays.
- A nuclear reaction is balanced in terms of both mass and energy. They involve only a rearrangement of electrons and do not involve changes in the nuclei. Here mass is not strictly conserved. Some of the mass is converted into energy, according to the equation E = mc2.
- Reactivity of an element towards nuclear reactions is nearly independent of the oxidation state of the element.
- The energy changes are far exceeding the energy changes in chemical reactions. These reactions are accompanied by large energy changes.
- In nuclear reactions, the energy involved is expressed in MeV (Million electron volts) per individual nucleus. This energy comes from the destruction of mass.
- A new element/isotope may be produced during the nuclear reaction. In these reactions, isotopes behave quite differently. For example, U-235 undergoes fission quietly readily but U-238 does not.
- The rate of a nuclear reaction is independent of temperature and pressure. Nuclear reactions involve the decomposition of the nucleus and have nothing to do with the electrons.
- In a nuclear reaction, the protons and neutrons react inside the nucleus and in chemical reactions, the electrons react outside the nucleus.
A nuclear reaction can be termed as either fission or fusion. In a chemical reaction, a substance is changed to one or more other substances because of the action of the electrons.