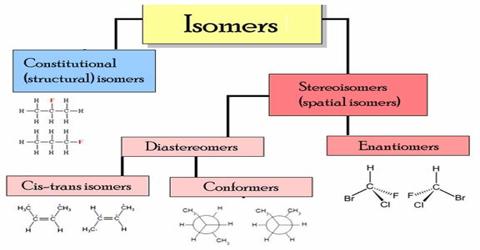
Dihaloalkane Isomerism: When dihaloalkanes are treated with excess of alcoholic KOH, alkynes are formed by the elimination of two molecules of halogen acids. Dihaloalkanes are of two types (i) 1,2-dihalides or vicinal dihalides (ii) 1,1-dihalides or gem dihalides.
Dihaloalkane shows two types of isomerism:
- Jem-dihalo: In this isomerism, two halogen atoms are attached together with the same carbon atom.
- Vicinal-dihalo: Two halogen atoms are attached together with the different carbon atoms.
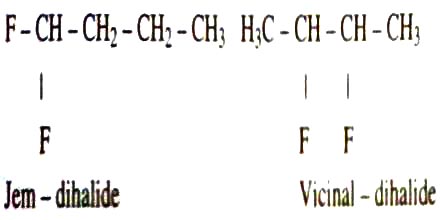
The reactivity of haloalkanes goes as follows: Iodoalkone > Bromoalkane > Chloroalkane
- Difluoro butane (C4H8F2) has 10 isomers