Effect of pressure on critical solution temperature: Liquid-Liquid Equilibria in Partially
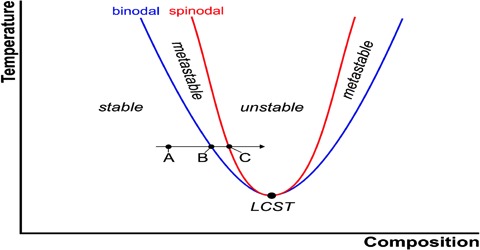
There are pairs of substances which are liquids at ordinary temperatures or whose melting points are low or are known to have limited mutual solubility within certain temperature limits. They are said to be partly miscible in the temperature limits and their solubility curves form an interesting aspect of the application of the phase rule. These substances become completely miscible beyond the temperature limits. These limits are sometimes attained on raising the temperature or sometimes on lowering the temperature. In some systems there are two limits, one higher and the other lower.
Effect of pressure on critical solution temperature
All systems so far described refer to constant pressure of the atmosphere or at any other constant pressure. Consequently, each t-c diagram is an isobar. However, if the pressure on such a system is changed the critical solution temperature is also found to change. The effect of pressure is governed by the Le Chatelier principle and the Clausius-Clapeyron equation. In general increase of pressure raises the lower CT and lowers the upper CST. Under appropriate pressures two CST’s may coincide with each other giving rise to complete miscibility at all temperatures. At one atmosphere pressure the two critical solution temperatures for water and secondary butyl alcohol are —12 °C and 114°C but at about 840 atmospheres the two CST’s are identical.
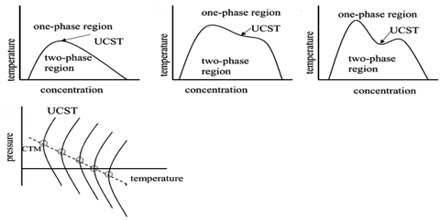
However, it should he mentioned that although the lower CST is always raised by increase of pressure the upper CST may be lowered or raised by increase of pressure. The latter behaviour is not very common.