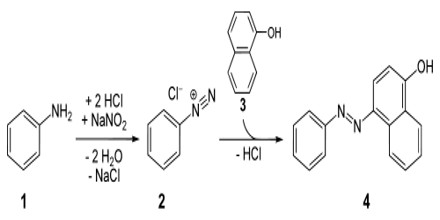
Formation of Azo dyes: Organic compounds become colorful due to the presence of some π- bonding group, e.g. nitroso (-NO) group, nitro group (-NO2), azo group (-N=N-) etc. So, the color-producing groups are known as a chromophore and the organic molecule having that colored group is known as chromogen. Azo dyes are poly-nuclear aromatic compounds having π-bonded diazo group as a chromophore.
Formation of Aniline Yellow: Benzene diazonium salt couples with aromatic primary amine in the presence of acid form azo dye (aniline yellow).
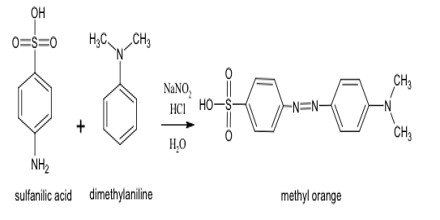
Formation of Butter Yellow: Benzene diazonium salt couples with tertiary amine (N, N- dimethylaniline) in the presence of acid and form azo dye (butter yellow).