Hydrogen-Oxygen Reaction
The gas phase reaction between hydrogen and oxygen to produce water is quite complex. Experimentally it is found that the reaction rate is dependent on pressure. When molecular hydrogen (H2) and oxygen (O2) are combined and allowed to react together, energy is released and the molecules of hydrogen and oxygen can combine to form either water or hydrogen peroxide.
A mixture of H2 and O2 in the ratio 2:1 reacts slowly in the temperature range of 450°- 6000 C as long as the pressure is very low. At a temperature of 550°C if the pressure exceeds about 2 mm Hg the mixture explodes. If, however, the reaction is started at an initial pressure of about 200 mm Hg the reaction is steady and proceeds at a measurable speed. On decreasing the pressure the rate decreases but below about 100 mm Hg pressure the reaction is again explosive. Thus at this temperature there is a region of pressure within which there is explosion, but above and below which the reaction rate is steady. This is shown schematically in Figure.
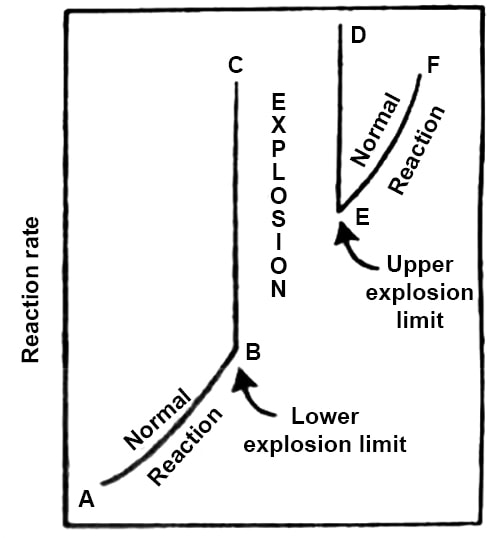
Fig: Explosion limits (third explosion limit not shown)
The proposed mechanism for the hydrogen – oxygen reaction is considered to have the following steps:
(1) H2 → 2H* Initiation
(2) H* + O2 → *OH + O* Branching
(3) *OH + H2 → *OH + H*
(4) *OH + H2 → H2O + H* Propagation
(5) H* + Wall → removal
(6) *OH + Wall → removal Termination
(7) *H + Wall → removal
Chain branching occurs in steps (2) and (3) and the reactions (5), (6) and (7) are the chain termination steps. The chain is propagated by step (4).
Other well-known examples of chain reactions air the nuclear fissions which when allowed to proceed uninhibited gives rise to explosions. The explosion of atomic bomb is of this type.