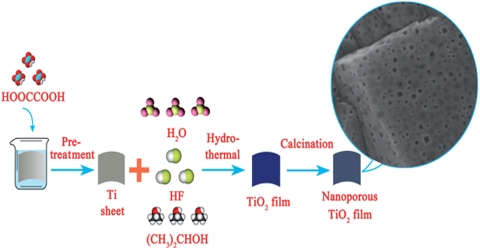
Hydrothermal method: When water is heated in a closed vessel (autoclave) the pressure inside is increased and water remains as liquid above its normal boiling point. This condition is called hydrothermal. The heating of reactants in ‘super-heated water’, (also known as supercritical water; >373 K) has received much attention in synthesising crystals, particularly in mineralogy.
Hydrothermal techniques can be used to synthesize a wide variety of materials
– zeolites and aluminophosphates
– optical materials like KTP (KTiOPO4)
– BaTiO3 (widely used ferroelectric)
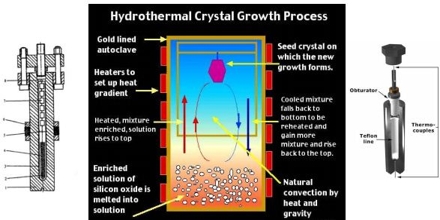
Hydrothermal synthesis of quartz
Uses a temperature gradient to dissolve the reactant at higher temperature, which is transported up the reaction tube by convection, then crystallizes out at a lower temperature.
Example: Hydrothermal synthesis of CrO2
Cr2O3 is the stable oxide of chromium at normal conditions.
Cr2O3 + CrO3 → 3CrO2
CrO3 → CrO2 + ½ O2
3 CrO3 + Cr2O3 → 5 CrO2 + O2