The Internal Energy of an Ideal Gas: Joule’s Experiments
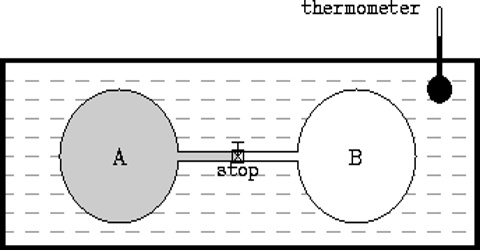
In an adiabatic expansion the work is done by the system on the surrounding at the expense of the internal energy. This was experimentally demonstrated by J. P. Joule in 1844. The experiments were per-formed in an apparatus described in Figure. Two similar copper vessels, one filled with an ‘ideal gas’ at a high pressure and the other evacuated, were used.
Fig: Joule’s Experiments
The vessels were connected by means of a stop-cock. These were then placed in a water bath, the temperature of which was recorded. When the stop-cork was open gas rushed from the high pressure vessel to the evacuated vessel. As a result the temperature of the vessel containing the gas under pressure fell, but that of the originally evacuated vessel rose by an equal amount. When equilibrium was established the temperature of the water bath was again recorded. There was no change in the temperature of the bath which means that there was neither absorption nor evolution of heat in the system.
Since the total volume of the system consisting of the two vessels had not changed, no external work was done against the atmosphere. Since q = 0 and w = 0, it follows from equation that ∆U must be zero. In other words, for an ideal gas the internal energy is independent of volume, i.e.,
(δU/δV)r = 0
This is regarded as a thermodynamic criterion for an ideal gas.