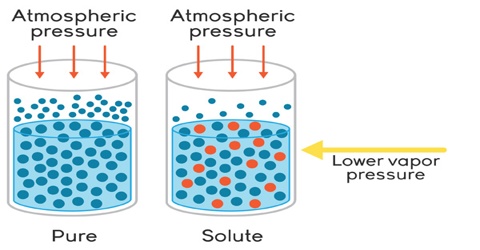
Interrelation between the Colligative Properties
The colligative properties of solutions are related with each other and mathematical relationships between these properties may be deduced. However, for the sake of brevity only the final results are given below:
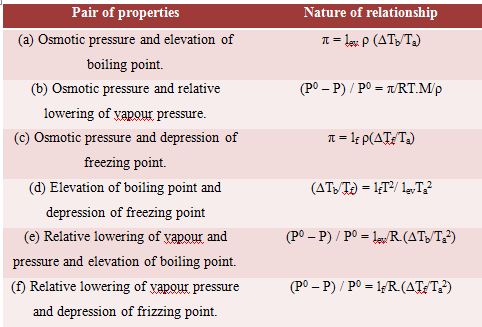
In the above relations, M is the molecular mass of the solvent and ρ is its density; lf and lev represent latent heat of fusion and latent heat of evaporation respectively.
It may be noted here that except osmotic pressure all other colligative properties are dependent on the solvent, e.g., extent at the elevation of boiling point or depression of freezing point depends on the solvent since Kb is related to the boiling point and latent heat of vaporization and Kf to freezing and latent heat of fusion. The relative lowering of vapour pressure depends on the solvent vapour pressure. But the osmotic Pressure is independent of the solvent. As long as the molar concentration remains the same the osmotic pressure remains unchanged at constant temperature.