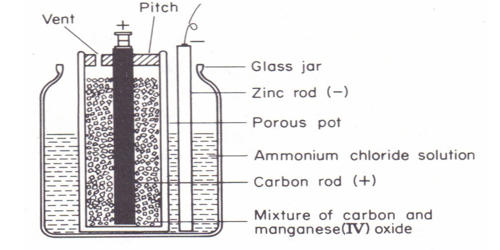
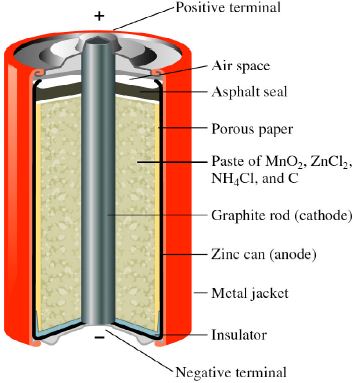
The Leclanche dry cell, or zinc-carbon dry cell, is a voltaic cell with a zinc can as the anode and a graphite rod in the center surrounded by a paste of manganese dioxide, ammonium and zinc chlorides, and carbon black, as the cathode. Leclanche cell is a primary cell, handy for sporadic use, with a positive anode of zinc encompassed by a mixture of manganese dioxide and powdered carbon in a pot, which is porous.
Variants of leclanche dry cell include:
- Zinc carbon (Carbon cathode)
- Zinc chloride (Ammonium chloride electrolyte replaced by zinc chloride)
- Alkaline manganese ( Ammonium chloride electrode replaced by potassium hydroxide)
The electrode reactions are as follows
Anode: Zn (s) → Zn2+ (aq) + 2e–
Cathode: 2NH4+ (aq) + 2MnO2 (s) + 2e– → Mn2O3 (s) + H2O (I) + 2NH3 (aq)
Applications of Leclanche dry cell: low-cost applications, toys, remote controls, flashlights, clocks, etc.
Working process
The process which generates power in a Leclanché cell starts when zinc particles on the surface of the anode oxidize, i.e. when zinc atoms surrender their valence electrons to end up becoming the positively charged particles. The zinc particles move far from the anode while leaving their electrons on its surface that makes the anode more negatively charged than the cathode. At the point when the cell is associated in an outer electrical circuit, the excess electrons on the zinc anode gush through the circuit to the cathode made up of carbon. This flow of electrons frames the electric current.
The whole of this process is accompanied by secondary reaction, wherein the negative hydroxide ions react with positive ammonium ions in the electrolyte of ammonium chloride to produce molecules of water and ammonia.
Practical Use
The Leclanche cell was utilized widely for telegraphy, electric bell and signaling work; and for work where the intermittent and low current was needed. The battery cell by Georges Leclanche proved out to be extremely advantageous in the early years of the telephones.
Characteristics
Primary cell with a nominal open-circuit voltage of 1.5 Volts produced in very high volumes.
Chemistry based on a zinc anode and a cathode/depolariser of manganese dioxide which absorbs the liberated hydrogen bubbles which would otherwise insulate the electrode from the electrolyte.
Advantages
- Inexpensive materials
- Low cost
- Suitable for a wide range of consumer applications
- Interchangeable with alkaline batteries
Shortcomings
- Propensity to leak
- The basic zinc-carbon battery has a lower energy density than the competing alkaline batteries
- Poor low-temperature performance. Do not function well in sub-zero temperatures.
- Not rechargeable
Applications
- General purpose, low-cost applications
- Toys
- Remote controls
- Flashlights
- Clocks
- Consumer applications.