Liquefaction of Gases — the Basic Principles
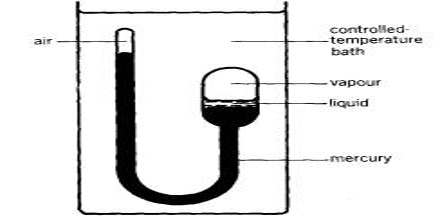
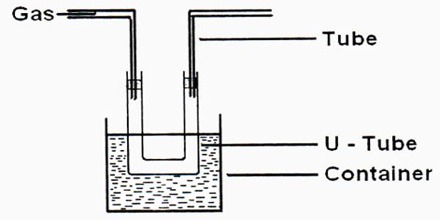
Liquefaction of gases is an exercise that had attracted the attention of scientists from very early times. As we know, increased pressure and lowering of temperature are used together or singly for the purpose. Gases such as nitrogen, oxygen, and methane require the use of very low temperatures to liquefy and store them at relatively low pressures. To achieve this, a whole range of cryogenic technologies has been developed to ensure the economical liquefaction of gases. In many cases, the investigators succeeded in liquefying easily liquefaction gases like NH3, SO2, Cl2, HCl, etc. Notable among the early work is due to Faraday (1823) who used a very simple device and was able to liquefy SO2, H2S, CO2, N2O, and NH3. He used an inverted U-tube (Figure) in one limb of which the gas was prepared from suitable reagents and in the other, the gas was liquefied under its own pressure with the help of cooling by immersion in a freezing mixture of ice and salt.
Thilorier (1834) used Faraday’s apparatus but made of wrought iron instead of glass, to liquefy CO2 in large quantities, and even to obtain solid CO2 by allowing partial evaporation of the liquid. He obtained the lowest temperature (-1100 C) stainable at the time by using a freezing mixture of solid CO2 and ether. The so-called permanent gases were not liquefied until Andrews discovered the critical phenomenon. His conclusion was that all gases could be liquefied provided these were cooled.
Below their respective crucial temperature; but as long as the temperature was above the critical value no amount of pressure would bring the gas to the liquid state.
Obviously gases like H2, N2, O2, etc. had critical temperatures lower than the lowest temperature attainable at the time. Thus followed an intense attempt at attaining very low temperature by the application of different physical principles.
Explanation
Liquefying a gas means the conversion of gas into a liquid. Liquefaction is the transformation of a gaseous substance into its liquid state. This change is the outcome of change in physical conditions like temperature, pressure, and volume. For example: When high pressure is applied to a gas, it gets compressed and when we lower its temperature it gets cooled.
Thomas Andrews investigated the complete relationship between volume- temperature and pressure of a substance in gaseous as well as liquid state by studying the behavior of carbon dioxide. In his experiment on CO2, Andrews came to a conclusion that at high temperatures, despite high pressure, the gases cannot be liquefied. After more research on this relationship, it was found that the high-temperature isotherms is similar to that of ideal gas but still under high pressure the gas cannot be liquefied. In the case of carbon dioxide, at 30.98° C, the gas started changing into a liquid. When the temperature of the gas is decreased then the curve shows deviation from the ideal one.