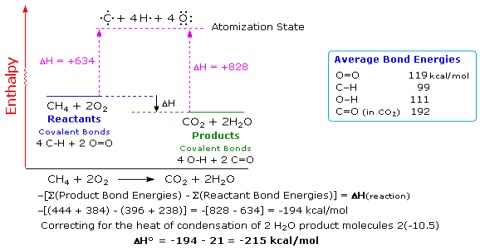
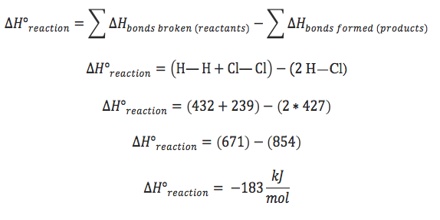
Standard enthalpy of reaction from average standard bond enthalpies
The average standard bond enthalpy data are useful in calculating the standard enthalpy of reactions. As stated earlier, in reactions involving covalent compounds bonds are broken and new bonds are formed. During chemical reactions, the bonds between atoms may break, reform or both to either absorb or discharge energy. The consequence is a change to the potential energy of the system. The heat absorbed or released from a system under constant pressure is known as enthalpy, and the change in enthalpy that results from a chemical reaction is the enthalpy of reaction. The standard enthalpy of reactions will, therefore, be the difference between the sum of the average standard bond enthalpies of bonds broken and the sum of the standard bond enthalpies of the bonds formed.
Mathematically,
∆H0R = ∑ (standard bond enthalpies of bonds broken) – ∑ (standard bond enthalpies of bonds formed)
The calculations of standard enthalpy of reactions from standard bond enthalpy data require two restrictions:
- The method applies only for covalent bonds.
- All species must be in the gas phase.