Sublimation in Triple Point
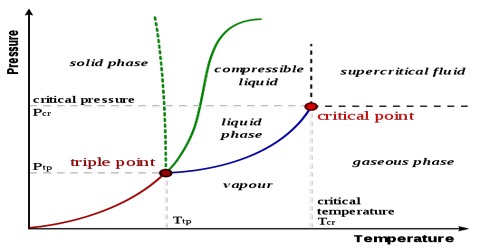
The process in which a solid is directly converted into vapour without passing through the intermediate liquid state is called Sublimation. The solid is said to have sublimed. Calomel, arsenious oxide, iodine etc. are known to sublime. It is known (curve OA in Figure) that ice can also sublime at temperature below 0.0098°C and a pressure 0.006 mm Hg. If, however, pressure of the solid at the triple point is above the atmospheric pressure the solid sublimes under ordinary conditions. Consider the phase diagram for carbon dioxide (Figure).
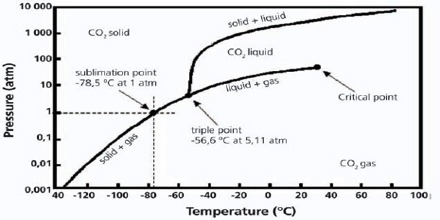
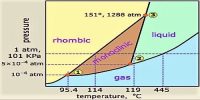
Fig: Phase diagram for carbon dioxide
The triple point temperature is – 570 C at 5.1 atmospheric pressure where liquid, solid and gaseous CO2 co-exist. At pressures below this value solid and vapour will remain in equilibrium at temperatures indicated by the line along DE. Therefore, the solid sublimes if heated at any pressure below 5.1 atmospheric pressure. This is why we see that solid CO2 (dry ice) sublimes at room temperature and pressure. It is not possible to obtain liquid CO2 simply by cooling at 1 atmospheric pressure. In order to obtain liquid CO2 it must be compressed to a pressure above 5.1 atmospheres and heated above temperature – 570 C such that the temperature and pressure are in the area FEM.
Solids, whose triple point pressures are below atmospheric pressure, pass on to the liquid state on heating, but if the working pressure can be rightly adjusted such solids can be made to sublime. On the other hand, a solid that sublimes under ordinary pressure can pass onto the liquid state if the pressure is raised above the triple point pressure. On quickly heating, iodine in a semi-closed vessel, it can be liquefied since under these conditions the pressure of the system rises above 90 mm of Hg which is the triple point pressure of iodine.