Unit of dipole moment
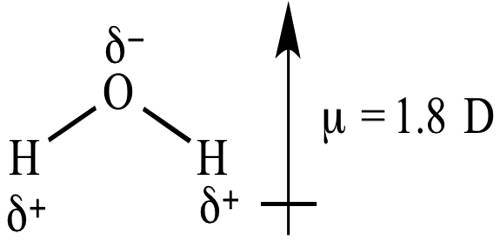
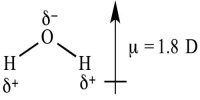
Dipole moments happen when there is a disconnection of charge. They can happen between two ions in an ionic bond or between atoms in a covalent bond; dipole moments occur from differences in electronegativity. A good example is the dipole moment of the water molecule. Molecules with mirror symmetry like oxygen, nitrogen, carbon dioxide, and carbon tetrachloride have no permanent dipole moments.
The unit of dipole moment is e.s.u.cm, the charge being expressed in an electrostatic unit (e.s.u) and the distance in cm, and dipole moment is usually expressed in Debye.
1 Debye = 10-18 e.s.u.cm
since the charge on one end is usually of the order of 10-10 e.s.u. and the distance between the centers of two atoms is of the order of 10-8 cm.
The larger the difference in electronegativities of bonded atoms, the larger the dipole moment. For example, NaCl has the highest dipole moment because it has an ionic bond (i.e. highest charge separation).