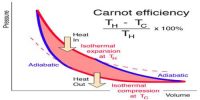
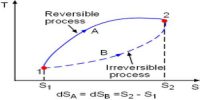
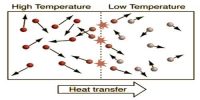
The first law of thermodynamics is a general statement of equivalence between work and heat. The second law of thermodynamics enables us to know whether a process which is allowed by first law of thermodynamics can actually occur or not. The second law of thermodynamics tells about the extent and direction of energy transformation.
Different scientists have stated this law in different ways to bring out its salient features.
(i) Kelvin’s statement
Kelvin’s statement of second law is based on his experience about the performance of heat engine. It is impossible to obtain a continuous supply of work from a body by cooling it to a temperature below the coldest of its surroundings.
(ii) Clausius statement
It is impossible for a self acting machine unaided by any external agency to transfer heat from a body at a lower temperature to another body at a higher temperature.
(iii) Kelvin – Planck’s statement
It is impossible to construct a heat engine operating in a cycle, which will extract heat from a reservoir and perform an equivalent amount of work.