Limitation of Bohr’s Atom Model
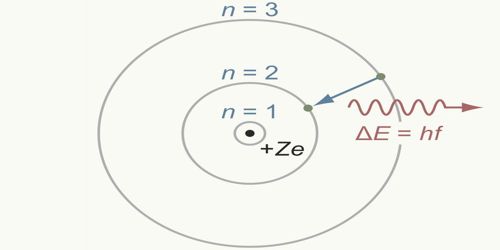
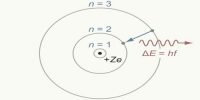
Bohr’s orbits are called permanent orbits because while orbiting these orbits electrons do not radiate energy. Although during orbiting electrons have acceleration, according to Bohr’s postulate electrons revolve without losing energy.
The Bohr Model was an important step in the development of atomic theory. However, it has several limitations.
- It is in violation of the Heisenberg Uncertainty Principle. The Bohr Model considers electrons to have both a known radius and orbit, which is impossible according to Heisenberg.
- It failed to explain the Stark effect when the spectral line gets split up into fine lines in the presence of an electric field.
- The Bohr Model is very limited in terms of size. Poor spectral predictions are obtained when larger atoms are in question.
- It cannot predict the relative intensities of spectral lines.
- The Bohr Model does not account for the fact that accelerating electrons do not emit electromagnetic radiation.
In explaining the origin of spectral lines and stability of atoms Bohr’s model has remarkable success, but some limitations of this model are also observed. Firstly, a probability of having an elliptical orbit, why electrons in the atom rotate in the circular orbit has not been explained in this model. From this, it appears that Bohr’s model is universal or complete. Secondly, hydrogen spectra are not unitary lines. From experiment, it has been proved that each line is the combination of a few fine lines of small energy difference. Bohr’s theory cannot explain this fine structure of hydrogen spectra.
Now, it is known that solar model does not express the picture of the atomic structure. Electron orbits as depicted in Bohr’s theory are not correct. Besides, electrons have wave property and charge distribution in different orbits as indicated by Bohr’s theory is different. Even after those limitations, Bohr’s theory has made a significant contribution to the development of modern physics.