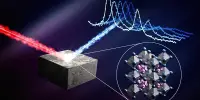
Researchers have made an important breakthrough in their quest to develop more sustainable luminescent materials and catalysts for converting sunlight into other forms of energy. They created a new class of compounds based on the inexpensive metal manganese that has promising properties previously only found in noble metal compounds.
Researchers at the University of Basel have reached a significant milestone in their quest to develop more sustainable luminescent materials and catalysts for converting sunlight into other forms of energy. They created a new class of compounds based on the inexpensive metal manganese that has promising properties previously only found in noble metal compounds.
Smartphone screens and catalysts for artificial photosynthesis, such as those used to produce fuel from sunlight, frequently contain extremely rare metals. Iridium, for example, is rarer than gold or platinum and is used in organic light-emitting diodes (OLEDs). Ruthenium, which is used in solar cells, is also one of the most scarce stable elements. Because of their scarcity, these metals are not only very expensive but also toxic in many compounds.
Researchers developed a new class of compounds, based on the cheap metal manganese, with promising properties that until now have primarily been found in noble metal compounds.
Now, a team led by Professor Oliver Wenger and his doctoral student Patrick Herr from the University of Basel has succeeded for the first time in producing luminescent manganese complexes that undergo the same reactions as ruthenium or iridium compounds when exposed to light. The research was published in the journal Nature Chemistry. Manganese has the advantage of being 900,000 times more abundant in the Earth’s crust than iridium, as well as being significantly less toxic and many times cheaper.
Rapid photochemistry
In terms of luminous efficiency, the new manganese complexes currently outperform iridium compounds. The light-driven reactions required for artificial photosynthesis, such as energy- and electron-transfer reactions, occur at high speeds. This is due to the new complexes’ unique structure, which causes an immediate charge transfer from manganese to its direct bonding partners upon light excitation. This complex design principle is already used in certain types of solar cells, though it has mostly focused on noble metal compounds and, on occasion, complexes based on the less noble metal copper.
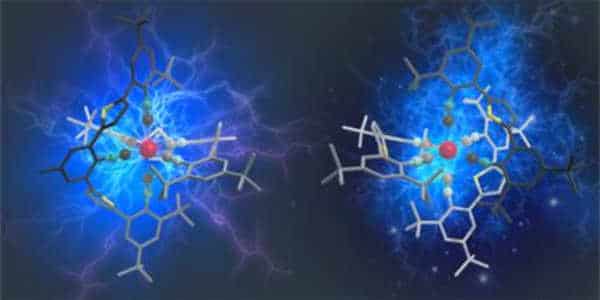
Preventing unwanted vibrations
Light energy absorption causes more distortion in complexes made of cheap metals than in complexes made of noble metals. As a result, the complexes begin to vibrate, and a large portion of the light energy absorbed is lost. The researchers were able to suppress these distortions and vibrations by incorporating custom-made molecular components into the complexes, forcing the manganese into a rigid environment. This design principle also improves the stability and resistance of the resulting compounds to decomposition processes.
No one has yet succeeded in creating molecular complexes with manganese that can glow in solution at room temperature and have these unique reaction properties, according to Wenger. “Patrick Herr and the postdocs involved made a significant breakthrough in this regard, one that opens up new opportunities beyond the field of noble metals.” Wenger and his colleagues hope to improve the luminescent properties of the new manganese complexes and anchor them on suitable semiconductor materials for use in solar cells in future research projects.
Other possible refinements include water-soluble manganese complexes that could potentially replace ruthenium or iridium compounds in photodynamic therapy for cancer treatment. The team was able to increase the stability of the resulting compounds and their resistance to decomposition processes by forcing manganese into a rigid environment. Until now, no one had succeeded in creating manganese molecular complexes that can glow in solution at room temperature and have these unique reaction properties.
Wenger and his colleagues stated in the paper that they hope to improve the luminescent properties of the new manganese complexes and anchor them on suitable semiconductor materials for use in solar cells in future research projects.