Standalone device to convert sunlight, carbon dioxide, and water into carbon-neutral fuel
Researchers have created a stand-alone system that transforms sunshine, carbon dioxide, and water into carbon-neutral fuel without needing any external components or electricity. The wireless device could theoretically be scaled up to build ‘energy farms,’ similar to solar farms, generating renewable fuels for the transformation sector and transporting away from fossil fuels.
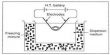
The system, created by a team from the University of Cambridge, is a major step towards achieving artificial photosynthesis-a mechanism that mimics the ability of plants to turn sunlight into electricity. It is built on advanced photosheet processing which transforms sunlight, carbon dioxide, and water into oxygen and formic acid-a storable fuel that can either be used directly or converted to hydrogen. The research team said that while the concept of using solar energy to turn CO2 into fuel is not new, it is a major challenge to do so without producing unnecessary by-products.
Researchers have developed a standalone, wireless device that can convert sunlight, carbon dioxide, and water into carbon-neutral fuel. The breakthrough technology is believed to be a significant step toward achieving artificial photosynthesis and quicker production of clean fuels.
The findings published in the journal Nature Energy represent a new approach for turning carbon dioxide into renewable fuels. The wireless system may be scaled up and used in electricity ‘farms’ similar to solar farms, generating renewable power using sunlight and water. Harvesting solar energy to turn carbon dioxide into power is a viable way to minimize carbon emissions and move away from fossil fuels. However, it is impossible to manufacture these renewable fuels without unnecessary by-products.
“It’s been difficult to achieve artificial photosynthesis with a high degree of selectivity, so that you’re converting as much of the sunlight as possible into the fuel you want, rather than be left with a lot of waste,” said Dr. Qian Wang, First Author of the Cambridge Department of Chemistry.
“In addition, storage of gaseous fuels and separation of by-products can be complicated — we want to get to the point where we can cleanly produce a liquid fuel that can also be easily stored and transported,” said Professor Erwin Reisner, senior author of the report.
In 2019, researchers from the Reisner group built a solar reactor based on the ‘artificial leaf’ concept, which also uses sunlight, carbon dioxide, and water to create a fuel known as syngas. The latest technology appears and behaves very similar to the artificial leaf, but functions in a different manner and contains formic acid.
While the artificial leaf used components from solar cells, the current technology does not need these components and relies solely on photocatalysis on the sheet to generate the so-called photocatalyst sheet. The sheets are made of semiconductor powders, which can be conveniently and cost-effectively prepared in large quantities.
In comparison, this modern technology is more durable and provides renewable fuel that is easier to store and has the ability to manufacture fuel goods on a scale. The test unit is 20 square centimeters in size, but the researchers say it should be reasonably easy to scale it up to many square meters. In addition, the formic acid may be accumulated in a solution, and chemically converted into various forms of gasoline.
“We were surprised how well it worked in terms of its selectivity — it produced almost no by-products,” Wang said. “Sometimes things don’t work as well as you expected, but this was a rare case where it actually worked better.”
The carbon dioxide converting cobalt-based catalyst is simple to produce and relatively stable. Although this system would be easier to scale than the artificial leaf, the performance needs to be increased before any commercial application can be contemplated. Researchers are working with a number of different catalysts to boost both stability and performance.
Present findings were obtained in collaboration with the team of Professor Kazunari Domen of the University of Tokyo, co-author of the analysis. Researchers are now working to better refine the system and increase performance. In addition, other catalysts are being investigated for the use of various solar fuels on the system. “We hope this technology will pave the way toward sustainable and practical solar fuel production,” said Reisner.