Chemistry
Explain Electronic Configuration of D-Block Elements
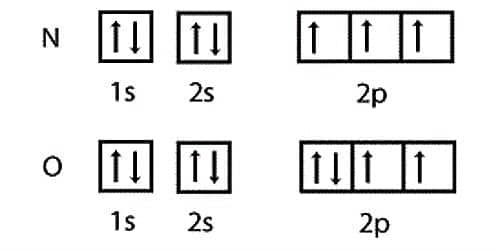
Electronic Configuration of D-Block Elements: In the transition elements, d-orbitals of penultimate shell are successively filled. The first transition series involves the filling of 3d…