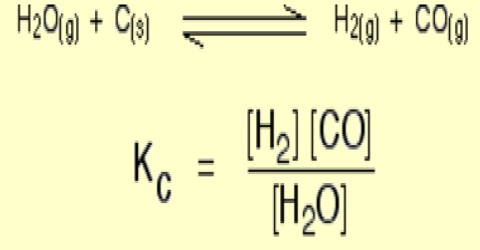
Chemistry
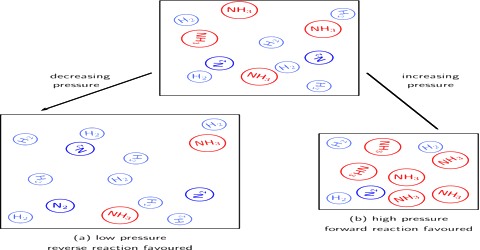
Applications of Principles of Chemical Equilibrium: Synthesis of Ammonia
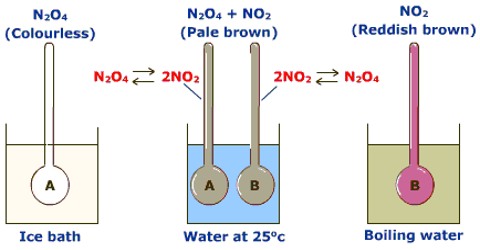
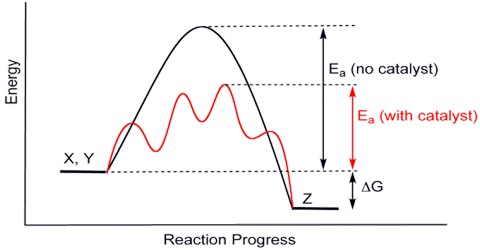
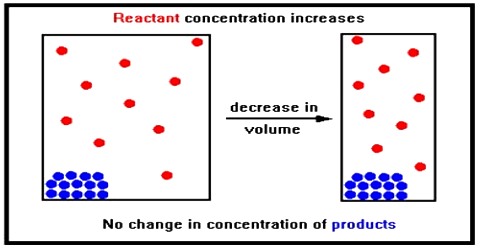
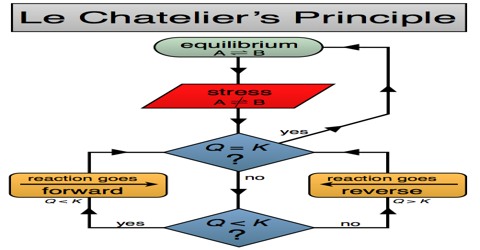
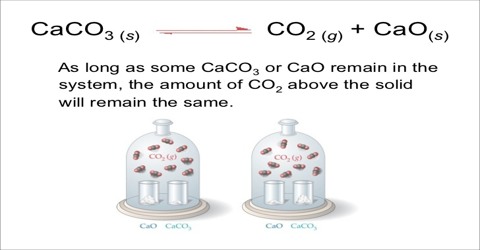
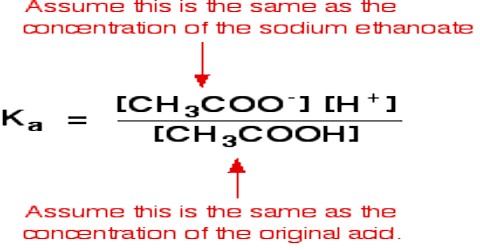
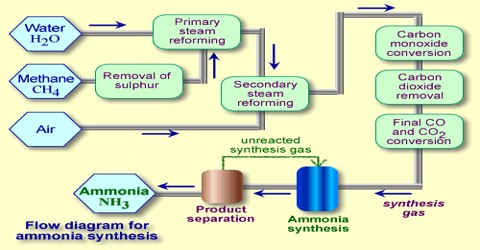
Applications of the Principles of Chemical Equilibrium to Reactions of Industrial Importance In industrial processes the primary objective is to maximize the yield of products…