Factors Influencing Equilibrium: The Principle of Le Chatelier
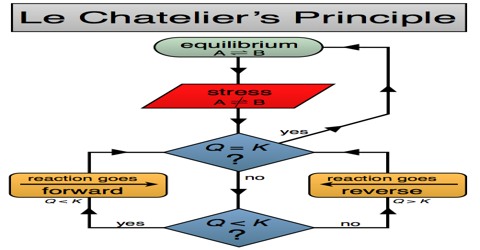
Le-Chatelier, a French Chemist, made a generalization to explain the effect of changes in concentration, temperature or pressure on the state of system in equilibrium. It states that: A chemical reaction at equilibrium will maintain this condition indefinitely unless disturbed by changes from outside.
Factors which may cause such disturbances are
(a) a change in concentration of any of the species present at equilibrium,
(b) a change in pressure in the case of equilibria where gases are present,
(c) change in temperature of the reaction, or
(d) addition of an inert substance.
The effect of these changes on the position of chemical equilibrium was qualitatively summarized by what is known as the principle of Le Chatelier. The principle states that-
“When a system is at equilibrium, a change in any one of the factors upon which the equilibrium depends will cause the equilibrium to shift in a direction such that the effect of the change is diminished.” Alternatively,
“If a stress is applied to a system at equilibrium, the position of equilibrium shifts in such a way that the effect of the stress is minimized.”
According to Le-Chatelier’s Principle, when the concentration of one of the substances in a system at equilibrium is increased, then the equilibrium will shift so as to use up the substance added. The resulting system will however, be still in equilibrium unless the change is very large. Equilibrium constant depends only on temperature but remains unchanged if any of the other factors is changed. The principle is of general utility and may also be applied to physical equilibria. This principle is also known as the principle of mobile equilibrium, clearly indicating that the equilibrium position can be easily shifted by changing conditions of the reaction. The equilibrium may be shifted to the right (product side) or to the left (reactant side), the direction of the change depending on the nature of the change brought into the system by altering the conditions that govern the equilibrium.