Criteria of Chemical Equilibrium
Whether in a system true equilibrium has been established or not need careful consideration. Several changes, both physical and chemical, appear at the first sight to be in a state of equilibrium but analysis reveals that true equilibrium had not been attained. For example, pure water, carefully freed from any suspended impurity and kept in an unstirred condition, can be easily cooled well below zero degrees centigrade without the formation of ice. A temperature of about – 50C can easily be reached without freezing taking place. The liquid water at temperatures below 00C is not in true equilibrium since suitable stirring, addition of a small quantity of ice or even sometime introduction of fine glass particle, scratching of the walls of the vessel etc. will cause rapid crystallization of ice and the temperature of the system quickly attains the true equilibrium value of 00C. Thus water below 0°C was in a state of false or unstable equilibrium, such unstable equilibrium is also called metastable equilibrium. A mixture of H2 and O2 at not too elevated temperatures is not in true equilibrium because the introduction of platinum black causes the reaction. A true equilibrium cannot be disturbed by a catalyst. Several reactions in which a precipitate should form instantaneously attain the equilibrium state only slowly. Such cases do not comprise true equilibrium. A true equilibrium, when established, will remain in that state for infinite time if the conditions governing the equilibrium, e.g., temperature, composition, pressure etc. are not altered. However, the time required for attainment of equilibrium may vary widely from fraction of seconds to months or years. The time depends on the relative values of the velocity constants of the forward and backward reactions. Once the true equilibrium is established, it will continue to remain so as long as it is not disturbed. Thus the equilibrium is permanent.
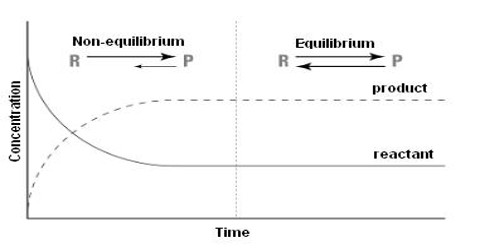
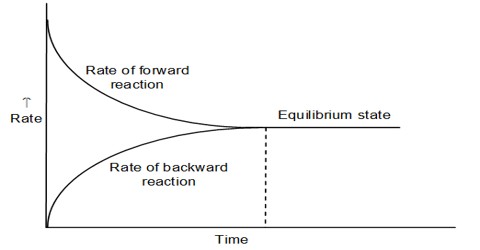
The statement and the physical concept of equilibrium, chemical or physical, clearly show that the equilibrium is dynamic. Thus equilibrium can be approached from either direction. Whether the equilibrium is attained via the forward reaction or the reverse reaction, the equilibrium constant remains the same as long as the conditions are not altered. The approachability of the equilibrium from both sides can be easily understood from Figure. When equilibrium has been established there will be no change in the concentrations of either the reactants or the products. This state can be ascertained from the analysis of the products or the reactants as shown in Figure 10.1. At this state, the forward and the reverse reactions proceed with the same velocity.
The very nature of the equilibrium constant suggests that a reversible reaction is not complete. If the reaction proceeds to completion, the equilibrium constant will assume a value of infinity or zero. Under both these conditions, the concept of chemical or physical equilibrium loses meaning and the equilibrium law cannot be used. Chemists, therefore, believe in dynamic nature of equilibrium and consequently in the incompleteness of chemical reactions. However, in many reactions, the equilibrium constants may be very large or very low. A range of 10-10 to 1010 is often met. Nevertheless values of infinity or zero are not acceptable to the physical chemists. Many reactions which appear to be unidirectional can be made to occur reversibly if suitable conditions are created. This concept has significant philosophical aspects ingrained in it.