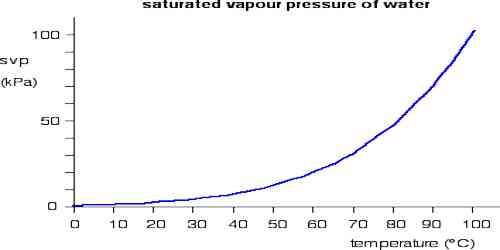
Characteristics of saturated vapour pressure
Basically, the vapor pressure is the partial pressure of liquid vapor (e.g. water vapor). Saturated vapor pressure is the vapor pressure which is in equilibrium with an open liquid surface. Therefore, it is also the pressure at which a liquid will vaporize for a given temperature. If the pressure to which the liquid is exposed is equal to the saturated vapor pressure for a given temperature, then the water will “boil”.
- A vapour in a closed space is said to be saturated at a given temperature if the pressure exerted by it is maximum at that temperature.
- It is created in a closed space.
- If there exists some vapour in contact with a liquid confined in a closed space, then that vapour is saturated vapour.
- Saturated vapour does not follow Boyle’s law and Charles’s law.
- By increasing temperature, a fixed amount of saturated vapour can be changed into unsaturated vapour.
- Saturated vapour remains in equilibrium with its liquid.
The process of evaporation in a closed container will proceed until there are as many molecules returning to the liquid as there are escaping. At this point, the vapour is said to be saturated, and the pressure of that vapor (usually expressed in mmHg) is called the saturated vapor pressure.