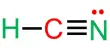The idea of exchanging electron, x2 (g) + 3y2 (g) → 2xy3
This reaction is known as oxidation reduction reaction,
The actual reaction,
N2 (g) + 3H2 (g) ↔ 2NH3
0 0 ↔ – 3 + 1
The oxidation number of H atom 0 → +1. So the oxidation number increases. The oxidation number of N atom 0 → – 3. So the oxidation number decreases. H2 releases 1 electron and N2 receive 3 electron. To equal the oxidation number H2 multiply by 3. In the reaction, oxidation -reduction occurs.