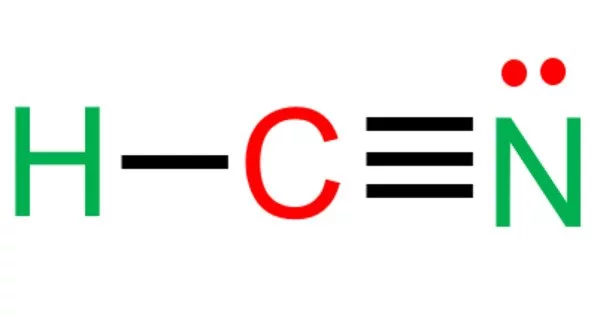
Hydrogen cyanide, also known as prussic acid, is a highly toxic chemical compound with the formula HCN and structural formula H-C≡N. It is a colorless, extremely poisonous, and flammable liquid that boils at 25.6 °C (78.1 °F), slightly above room temperature. HCN is manufactured on a large scale and is a valuable precursor to a wide range of chemical compounds, from polymers to pharmaceuticals.
Large-scale applications include the production of potassium cyanide and adiponitrile, which are used in the mining and plastics industries, respectively. Because of its volatile nature, it is more toxic than solid cyanide compounds.
Properties
- Chemical formula: HCN
- Molar mass: 27.0253 g/mol
- Appearance: Colorless liquid or gas
- Odor: Almond-like
- Density: 0.6876 g/cm3
- Melting point: −13.29 °C (8.08 °F; 259.86 K)
- Boiling point: 26 °C (79 °F; 299 K)
- Solubility in water: Miscible
- Solubility in ethanol: Miscible
- Vapor pressure: 100 kPa (25 °C)
Chemical properties
Hydrogen cyanide will react with alkenes under catalysis of nickel complexes. This reaction is called hydrocyanation.
RCH=CH2 + HCN → RCH2-CH2-CN
Four molecules of HCN will tetramerize into diaminomaleonitrile, which can be converted to various purines.
Occurrence
Hydrogen cyanide can be found naturally in plants such as apple, peach, and almond seeds. It can also be synthesized synthetically through a variety of chemical processes.
HCN can be obtained from pitted fruits such as cherries, apricots, apples, and bitter almonds, which are used to make almond oil and flavoring. Many of these pits contain trace amounts of cyanohydrins like mandelonitrile and amygdalin, which release hydrogen cyanide slowly. One hundred grams of crushed apple seeds contain approximately 70 mg of HCN. Cassava plant “bitter” roots can contain up to 1 gram of HCN per kilogram.
Uses
Despite its toxicity, hydrogen cyanide has a few industrial applications. It is used to make synthetic fibers, dyes, plastics, and pharmaceuticals. It can also be used as a fumigant, particularly in pest control.
Toxicity
Hydrogen cyanide is a highly toxic substance. It impairs the body’s ability to use oxygen, resulting in cellular asphyxiation. If inhaled or ingested, even low concentrations can be fatal. High concentrations can be fatal in a matter of minutes.