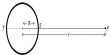
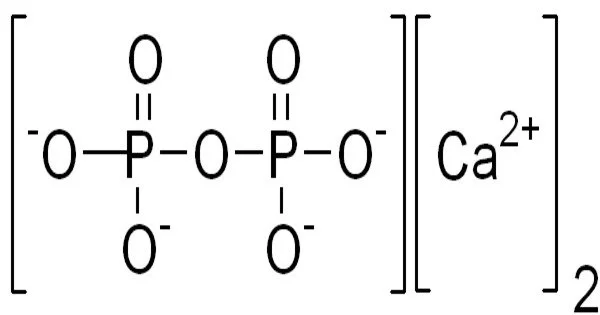Calcium pyrophosphate (Ca2P2O7) is a chemical compound composed of calcium cations (Ca2+) and pyrophosphate anions (P2O74-). It is also known as dicalcium pyrophosphate (DCPP) and is commonly used as a food additive, particularly in baking powders as a leavening agent.
Calcium pyrophosphate is an insoluble calcium salt that contains the pyrophosphate anion. There are several reported forms, including an anhydrous form, a dihydrate, Ca2P2O7•2H2O, and a tetrahydrate, Ca2P2O7•4H2O. In cases of calcium pyrophosphate deposition disease (pseudo gout), which has symptoms similar to gout, dihydrate crystal deposition in cartilage is responsible for severe joint pain. Because of its insolubility and nonreactivity to fluoride, Ca2P2O7 is commonly used as a mild abrasive agent in toothpastes.
Properties
- Physical appearance: It is a white, odorless powder that is insoluble in water.
- Melting point: The melting point is around 1240°C.
- Density: The density is around 2.72 g/cm3.
- Solubility: It is insoluble in water but slightly soluble in dilute acids.
- Stability: It is stable under normal conditions, but it can decompose at high temperatures to release phosphorus pentoxide and calcium oxide.
Uses
Calcium pyrophosphate is mainly used as a food additive (E450) to control acidity, as a stabilizer in the production of non-dairy creamers, and as a polishing agent for teeth.
In addition to its use as a food additive, calcium pyrophosphate can form in the body, resulting in a medical condition known as calcium pyrophosphate deposition disease (CPPD). CPPD develops when calcium pyrophosphate crystals build up in the joints, causing pain and inflammation. It is often referred to as pseudogout because it can mimic the symptoms of gout, another type of crystal-induced arthritis.
Medical significance
Calcium pyrophosphate deposition disease (CPPD), also known as pseudogout, is a medical condition that results from the deposition of calcium pyrophosphate crystals in joints, causing joint pain and inflammation.
However, CPP can also accumulate in joints and soft tissues, leading to a condition called calcium pyrophosphate deposition (CPPD) disease. CPPD disease is characterized by the formation of CPP crystals in the affected tissues, which can cause inflammation and damage. CPPD disease can affect different joints in the body, including the knees, wrists, and hips, and can cause symptoms such as pain, swelling, and stiffness.
CPPD can be diagnosed through the use of imaging techniques such as X-rays or MRI, as well as joint fluid analysis. Treatment may include nonsteroidal anti-inflammatory drugs (NSAIDs), colchicine, or corticosteroids to reduce inflammation and manage symptoms. In some cases, joint aspiration or surgery may be necessary to remove accumulated crystals.