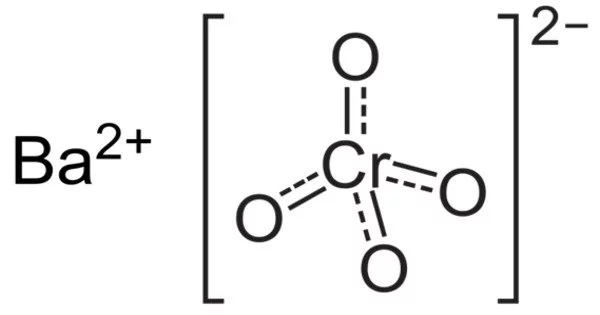
The IUPAC name for barium chromate, barium tetraoxochromate(VI), is a yellow sand-like powder with the formula BaCrO4. It acts as an oxidizer. The chromium in this compound has an oxidation state of +6. It is a well-known oxidizing agent that, when heated, produces a green flame due to the presence of barium ions. When heated, the Barium ions in the compound produce green flames.
It is widely used as an oxidizer as well as a burn rate modifier in many pyrotechnic compositions, particularly delay compositions (a substance that produces an effect by light, sound, heat, gas, and smoke as a result of self-sustaining exothermic reactions).
Properties
- Chemical formula: BaCrO4
- Molar mass: 253.37 g/mol
- Appearance: yellow powder, darkens upon heating
- Density: 4.498 g/cm3
- Melting point: 210 °C (410 °F; 483 K) (decomposes)
- Solubility in water: 0.2775 mg/100 mL (20 °C)
- Solubility product (Ksp): 1.17 × 10−10
- Solubility: soluble in strong acids

Production
Barium Chromate can be produced by the precipitation reaction of Barium Chloride (BaCl2) and Sodium Chromate (Na2CrO4). The reaction occurs in the following way:
BaCl2 + Na2CrO4 → BaCrO4 ↓ +2 NaCl
This reaction produces BaCrO4 precipitation which is then collected by filtration. Sodium Chloride is also produced (NaCl) in this reaction.
History
Jordan was the first country to discover naturally occurring barium chromate. The brown crystals discovered perched on host rocks were named hashemite in honor of Jordan’s Hashemite Kingdom. Hashemite crystals are typically less than 1mm in length and range in color from light yellowish-brown to a darker greenish-brown.
The hashemite crystals are not made entirely of barium chromate, but also contain a trace of sulfur. Hashemite, BaSO4, was discovered to be an isostructural chromate analog of baryte.
Common Uses
Barium chromate has been discovered to be useful in a variety of applications. The compound is frequently used as a chromium ion carrier. The use of barium chromate as a sulfate scavenger in chromium electroplating baths is one example. The chromium concentration in the bath will gradually decrease until the bath is no longer functional. By increasing the chromic acid concentration, barium chromate extends the life of the bath.
Because barium chromate is an oxidizing agent, it can be used to modify the burn rate of pyrotechnic compositions. It’s particularly useful in delay compositions like delay fuses. When zinc-alloy electroplating surfaces, it is used as a corrosion inhibitive pigment.
Barium chromate, when mixed with solid fumaric acid, can be used to remove impurities and residual moisture from organic dry-cleaning solvents or petroleum fuels. It is also used in the formulation of an alkane dehydrogenation catalyst.
Paints have also been colored with barium. Lemon yellow pigment was frequently made up of barium chromate mixed with lead sulfate. Lemon yellow was not commonly used in oil painting due to its low tinting strength. Lemon yellow was used in the paintings of Pierre-Auguste Renoir and Claude Monet.
Safety
Barium chromate is poisonous. Chromates are carcinogens when pulverized and inhaled. Many human organs, including the kidney, lungs, blood, nervous system, liver, and mucus membrane, can be damaged.