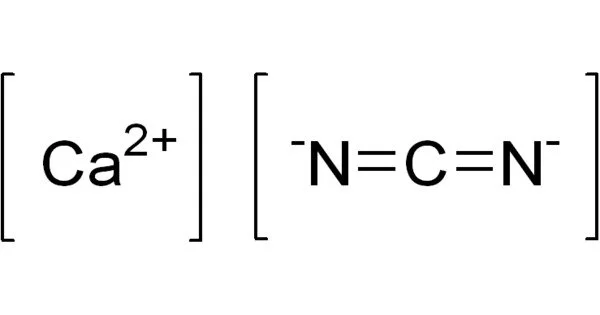The inorganic compound with the formula CaCN2 is calcium cyanamide. It is the cyanamide anion’s calcium salt. This chemical, known commercially as nitrolime, is used as fertilizer. Adolph Frank and Nikodem Caro were the first to synthesize it in 1898. (Frank–Caro process).
Calcium cyanamide is used in the production of fertilizer, pesticides, and other chemicals. It is irritating to humans’ eyes, skin, and respiratory tract.
Properties
It is a colorless to gray, odorless solid. It may cause illness from ingestion. It may irritate the skin. If exposed to water or high temperatures, calcium cyanamide may generate toxic and flammable fumes. Used to make pesticides and in fertilizers.
- Chemical formula: CaCN2
- Molar mass: 80.102 g/mol
- Appearance: White solid (Often gray or black from impurities)
- Odor: odorless
- Density: 2.29 g/cm3
- Melting point: 1,340 °C (2,440 °F; 1,610 K)
- Boiling point: 1,150 to 1,200 °C (2,100 to 2,190 °F; 1,420 to 1,470 K) (sublimes)
- Solubility in water: Reacts

Production
Calcium cyanamide is prepared from calcium carbide. The carbide powder is heated at about 1000 °C in an electric furnace into which nitrogen is passed for several hours. The product is cooled to ambient temperatures and any unreacted carbide is leached out cautiously with water.
CaC2 + N2 → CaCN2 + C (ΔHo
f = –69.0 kcal/mol at 25 °C)
It crystallizes in hexagonal crystal system with space group R3m and lattice constants a = 3.67 Å, c = 14.85 Å.
Calcium cyanamide was first produced commercially around 1900 as a fertilizer. The process of making calcium cyanamide involves three raw materials—coke, coal, and limestone— plus nitrogen. The limestone (calcium carbonate) is burned with coal to produce calcium oxide. The calcium oxide is then allowed to react with amorphous carbon in the furnace at 2000°C with the formation of calcium carbide (CaC2). Finely powdered calcium carbide is heated to 1000°C in an electric furnace into which pure nitrogen is passed. It is then removed and uncombined calcium carbide removed by leaching.
Uses
Calcium cyanamide is used as a fertilizer, defoliant, herbicide, fungicide, and pesticide; in the manufacture and refining of iron; and in the manufacture of calcium cyanide, melamine, and dicyandiamide
The main use of calcium cyanamide is in agriculture as a fertilizer. In contact with water, it decomposes and liberates ammonia:
CaCN2 + 3 H2O → 2 NH3 + CaCO3
It was used to produce sodium cyanide by fusing with sodium carbonate:
CaCN2 + Na2CO3 + 2 C → 2 NaCN + CaO + 2 CO
Sodium cyanide is used in cyanide process in gold mining. It can also be used in the preparation of calcium cyanide and melamine. Through hydrolysis in the presence of carbon dioxide, calcium cyanamide produces cyanamide:
CaCN2 + H2O + CO2 → CaCO3 + H2NCN
The conversion is conducted in slurries. For this reason, most commercial calcium cyanamide is sold as an aqueous solution. Thiourea can be produced by the reaction of hydrogen sulfide with calcium cyanamide in the presence of carbon dioxide.
Calcium cyanamide is also used as a wire-fed alloy in steelmaking to introduce nitrogen into the steel.
Safety
The substance can cause alcohol intolerance, before or after the consumption of alcohol. Calcium cyanamide is irritating to the eyes, skin, and respiratory tract in humans. Acute inhalation exposure may cause gastritis, rhinitis, pharyngitis, laryngitis, and tracheobronchitis. Acute oral exposure of humans may cause a vasomotor reaction, resulting in intense localized erythematous flushing of the face, upper body, and arms, with headache, dizziness, fatigue, vertigo, congestion of the mucosa, nausea, and vomiting also reported.