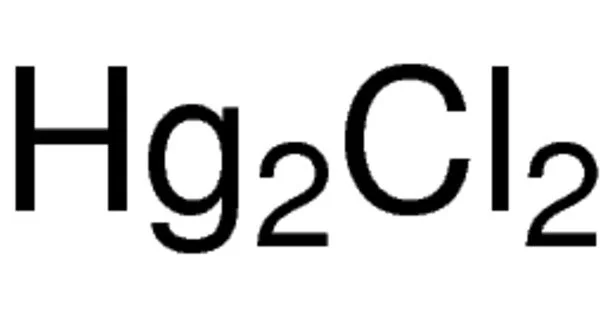Mercury(I) bromide, also known as mercurous bromide, is a chemical compound with the formula Hg2Br2 that combines mercury and bromine. It’s a mercury coordination entity made up of both mercury and bromine. When heated, it changes color from white to yellow, and when exposed to ultraviolet light, it fluoresces a salmon color. It can be used in acousto-optical devices.
Kuzminite is a very rare mineral with the chemical formula Hg2(Br,Cl)2. It is used in the pharmaceutical industry as well as an analytical reagent.
Properties
Mercurous bromide is a white, odorless powder. It darkens when exposed to light. It is sublime at high temperatures. It is produced by oxidizing elemental mercury with elemental bromine or by adding sodium bromide to a solution of mercury(I) nitrate. Mercury is a heavy, silvery d-block metal and one of six elements that are liquid at or near room temperature and pressure. It is a naturally occurring substance that combines with other elements such as chlorine, sulfur, or oxygen to form inorganic mercury compounds (salts).
- Chemical formula: Hg2Br2
- Molar mass: 560.99 g/mol
- Appearance: white to yellow tetragonal crystals
- Odor: odorless
- Density: 7.307 g/cm3, solid
- Melting point: 405 °C (761 °F; 678 K)
- Boiling point: ~ 390 °C (734 °F; 663 K) sublimes
- Solubility in water: 3.9 x 10−5 g/100 mL
- Solubility product (Ksp): 6.4×10-23
- Solubility: insoluble in ether, acetone, alcohol
- Molecular shape: linear

Reactions
Mercury(I) bromide is made by oxidizing elemental mercury with elemental bromine or by adding sodium bromide to a solution of mercury(I) nitrate. It decomposes into mercury(II) bromide and elemental mercury.
Structure
In common with other Hg(I) (mercurous) compounds that contain linear X-Hg-Hg-X units, Hg2Br2 contains linear BrHg2Br units with a Hg-Hg bond length of 249 pm (Hg-Hg in the metal is 300 pm) and a Hg-Br bond length of 271 pm. The overall coordination of each Hg atom is octahedral because, in addition to the two nearest neighbors, there are four other Br atoms at 332 pm. Although it is a molecular compound, it is commonly written as Hg22+ 2Br-.
Health Hazard
TOXIC; inhaling, ingesting, or coming into contact with the material may result in severe injury or death. Contact with molten material can result in severe burns to the skin and eyes. Any skin contact should be avoided. Contact or inhalation effects may be delayed. Fire can emit irritant, corrosive, and/or toxic gases. Firefighting or dilution water runoff can be corrosive and/or toxic, resulting in pollution.