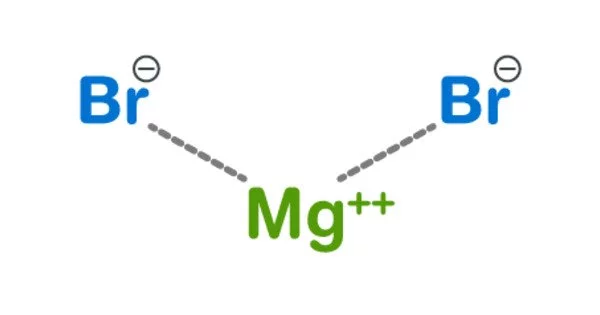
Magnesium bromide, with the chemical formula MgBr2, is a magnesium and bromine compound. It is a crystalline solid that is white and deliquescent. It comes in two different forms: anhydrous and hexahydrate. It is frequently used to treat nervous disorders as a mild sedative and anticonvulsant. It can be used as a catalyst in a variety of reactions. It is soluble in water and somewhat soluble in alcohol. It can be found in trace amounts in minerals such as bischofite and carnallite, as well as sea water such as the Dead Sea.
Properties
Magnesium bromide appears as white hygroscopic crystals in the anhydrous form and as colorless monoclinic crystals in the hexahydrate form.
- Chemical formula: MgBr2 (anhydrous); MgBr2·6H2O (hexahydrate)
- Molar mass: 184.113 g/mol (anhydrous); 292.204 g/mol (hexahydrate)
- Appearance: white hygroscopic hexagonal crystals (anhydrous); colorless monoclinic crystals (hexahydrate)
- Density: 3.72 g/cm3 (anhydrous); 2.07 g/cm3 (hexahydrate)
- Melting point: 711 °C (1,312 °F; 984 K) 172.4 °C, decomposes (hexahydrate)
- Boiling point: 1,250 °C (2,280 °F; 1,520 K)
- Solubility in water: 102 g/(100 mL) (anhydrous); 316 g/(100 mL) (0 °C, hexahydrate)
- Solubility: ethanol: 6.9 g/(100 mL); methanol: 21.8 g/(100 mL)
- Crystal structure: Rhombohedral, hP3

Preparation
It can be prepared by the following method
- Treating hydrobromic acid (HBr) with magnesium oxide (MgO) and crystallizing the product.
- It can also be synthesized by reacting magnesium carbonate (MgCO3) and hydrobromic acids (HBr).
On completing this it is evaporated and the solid leftover is collected. It is soluble in water as well as alcohol. It is naturally found in some minerals such as carnallite and bischofite.
Synthesis
By reacting hydrobromic acid with magnesium oxide and crystallizing the product, magnesium bromide can be synthesized. It can also be made by combining magnesium carbonate and hydrobromic acids and collecting the solid that remains after evaporation.
Uses
Magnesium bromide is used as a catalyst in a variety of reactions, the first of which is a solvent-free one-pot synthesis of dihydropyrimidinones, which are frequently used in pharmaceuticals such as calcium channel blockers and HIVgp-120-CD4 inhibitors. It has also been used as a sedative. Magnesium bromide, in conjunction with CH2Cl2, catalyzes a reaction that results in specific symmetry and chiral centers via alkene hydrogenation.
- It is used as a tranquilizer
- It is used as a catalyst
- It is used to analyze regiospecific of triglycerols.
- It is widely used as an anticonvulsant for the treatment of nervous disorders.
- It is used as a mild sedative.