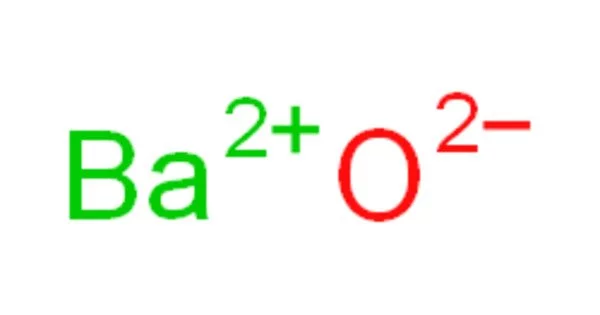Barium oxide, also known as baria, is a white hygroscopic non-flammable compound with the formula BaO. It’s a non-flammable yellowish-white material. It has the chemical formula BaO and is also hygroscopic, which means it readily absorbs moisture from the air. It has a cubic structure and is used in cathode ray tubes, crown glass, and catalysts. It is harmful to human skin and causes irritation when consumed in large quantities. Excessive consumption of barium oxide may result in death.
Properties
- Molecular weight: 153.326 g/mol
- Density: 5.72 g/cm3
- Boiling point: ~ 2,000 °C
- Melting point: 1,923 °C
- Crystal structure: Cubic
- Solubility in water at 20°C: 3.48 g/100ml
- Magnetic susceptibility: -29.1×10−6 cm3/mol
- Flashpoint: Non-flammable
- Specific Gravity: 5.72

Preparation
Barium oxide is produced by heating barium carbonate at temperatures ranging from 1000 to 1450 °C. It can also be made by thermally decomposing barium nitrate. Similarly, it is frequently formed by the decomposition of other barium salts.
It is made by heating barium carbonate in the presence of coke, carbon black, or tar, or by thermal decomposition of barium nitrate.
2Ba + O2 → 2BaO
BaCO3 → BaO + CO2
Barium oxide has also been synthesized by reacting barium chloride, BaCl2, with ammonia, NH3, and precipitating it with deionized water. This is one of several methods for producing barium oxide that can only be done in a laboratory under controlled conditions.
Commercially, the extraction of barium oxide from barium carbonate poses extraction challenges. This is due to the fact that it is frequently contaminated with excess carbon, which is usually added to prevent barium oxide from converting to barium peroxide. The presence of excess carbon causes the mixture of carbon and barium oxide to appear black. Removing this excess carbon simply by heating the mix is not feasible as that would cause a reaction with the barium oxide and regeneration of barium carbonate, essentially reversing the progress made.
Uses
Barium oxide is used to coat hot cathodes, such as those found in cathode ray tubes. It took the place of lead(II) oxide in the manufacture of certain types of glass, such as optical crown glass. Lead oxide increased the refractive index, but it also increased the dispersive power, which barium oxide does not change. In addition, barium oxide is used as an ethoxylation catalyst in the reaction of ethylene oxide and alcohols, which occurs between 150 and 200 °C.
It also provides pure oxygen through heat fluctuation. It easily oxidizes to BaO2 via the formation of a peroxide ion. The complete peroxidation of BaO to BaO2 occurs at moderate temperatures, but the increased entropy of the O2 molecule at high temperatures means that BaO2 decomposes to O2 and BaO at 1175K. Before air separation became the dominant method at the turn of the twentieth century, the reaction was used on a large scale to produce oxygen. The Brin process was named after its creators.
Safety issues
Irritating is barium oxide. When it comes into contact with the skin or the eyes, or is inhaled, it causes pain and redness. When ingested, however, it becomes more dangerous. It can cause nausea and diarrhea, as well as muscle paralysis and cardiac arrhythmias, as well as death. If ingested, seek medical attention as soon as possible.
Barium oxide should not be released into the environment because it is toxic to aquatic organisms. It is a toxic and corrosive compound that is non-combustible and sensitive to water.