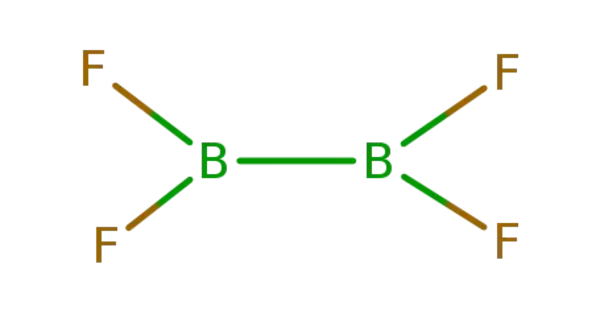
Diboron tetrafluoride is an inorganic compound with the formula (BF2)2. The compound is a colorless gas with a halflife of days at room temperature. It is the most stable of the diboron tetrahalides.
Diboron tetrafluoride is used in the production of high-quality optical glass as a dopant to improve the clarity and color purity of the glass. It is also used as an etching agent in the fabrication of semiconductor devices.
Properties
Diboron tetrafluoride is a molecule consisting of two boron atoms and four fluorine atoms. It is a colorless, toxic gas with a pungent odor.
- Chemical formula: B2F4
- Molar mass: 97.616 g/mol
- Appearance: Colorless gas
- Density: 4.3 kg/m3 (gas)
- Melting point: −56 °C (−69 °F; 217 K)
- Boiling point: −34 °C (−29 °F; 239 K)
- Heat capacity (C): 79.1 J/mol K
Structure and bonding
Diboron tetrafluoride is a planar molecule with a B-B bond distance of 172 pm. Although it is electron-deficient, the unsaturated boron centers are stabilized by pi-bonding with the terminal fluoride ligands. The compound is isoelectronic with oxalate. The compound is a covalent compound since boron and fluorine are both nonmetals. The compound is named by writing the names of the elements with the appropriate prefix indicating the number of each element in the compound.
Synthesis and reactions
Diboron tetrafluoride can be formed by treating boron monofluoride with boron trifluoride at low temperatures, taking care not to form higher polymers.
Addition of diboron tetrafluoride to Vaska’s complex was employed to produce an early example of a transition metal boryl complex:
2 B2F4 + IrCl(CO)(PPh3)2 → Ir(BF2)3(CO)(PPh3)2 + ClBF2
Application
It is a white, odorless solid that is insoluble in water. Diboron tetrafluoride is produced by the reaction of boron trioxide and fluorine gas at high temperatures. It is used as a Lewis acid in organic synthesis.