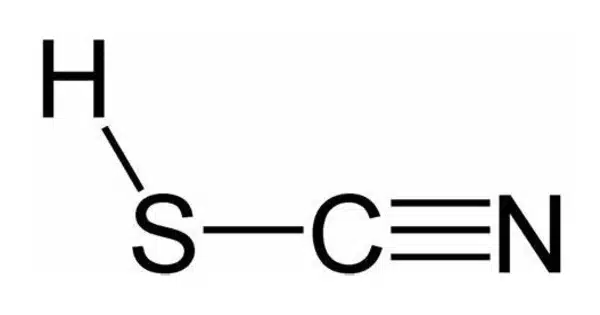
Thiocyanic acid is a chemical molecule with the formula HSCN and the structural formula H−S−C≡N, and it exists as a tautomer with isothiocyanic acid (H−N=C=S). It is a colorless liquid with a strong odor in its pure form. The iso-form predominates, with the vapor phase containing approximately 95% isothiocyanic acid.
It is a fairly strong acid, having a pKa of 1.1 at 20 °C and a zero ionic strength extrapolation. Because of its instability and proclivity to disintegrate, it is rarely found in its free acid state. It is more commonly encountered in the form of its salts, known as thiocyanates. Potassium thiocyanate (KSCN) and sodium thiocyanate (NaSCN) are two common thiocyanates.
A triple bond between carbon and nitrogen is expected for HSCN. It has been spectroscopically seen but not isolated as a pure material. Thiocyanates are the salts and esters of thiocyanic acid. The salts are made up of the thiocyanate ion (−SCN) and a metal cation (for example, potassium thiocyanate, KSCN). Thiocyanic acid esters have the general structure R-SCN.
Properties
Isothiocyanic acid, HNCS, is a Lewis acid whose free energy, enthalpy, and entropy changes for its 1:1 association with a variety of Lewis bases in carbon tetrachloride solution at 25 °C have been reported. HNCS acceptor properties are discussed in the ECW model.
- Chemical formula: CHNS
- Molar mass: 59.09 g·mol−1
- Appearance: colorless, oily liquid
- Odor: pungent
- Density: 2.04 g/cm3
- Melting point: 5 °C (41 °F; 278 K)
- Solubility in water: Miscible
- Solubility: soluble in ethanol, diethyl ether
- log P: 0.429
- Acidity (pKa): 0.926
- Basicity (pKb): 13.071
Applications
- Analytical Chemistry: These are used as reagents in analytical chemistry to test for the presence of certain metal ions. For example, the formation of a red-colored complex with iron(III) ions is used as a qualitative test for iron.
- Pharmaceuticals: These are sometimes used in the synthesis of pharmaceuticals and organic compounds.
- Photography: In traditional photography, ammonium, and sodium thiocyanate are used in fixing baths to remove unexposed silver halide from photographic paper or film.
- Galvanization: In the field of metal finishing, thiocyanates are used to remove oxide layers from the surfaces of metals before they are galvanized.
- Biological Role: Thiocyanates can also be found in biological systems. For example, they are produced by the body when metabolizing certain substances and can be detected in biological fluids such as saliva.