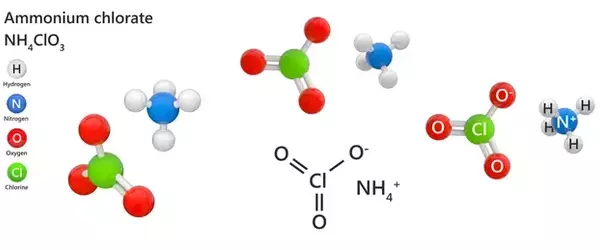
Ammonium chlorate has the formula NH4ClO3 and is an inorganic chemical. It is a very unstable salt that is primarily utilized in the production of explosives. It is exceedingly unstable in its pure state and can quickly detonate. It is a potent oxidant, but its employment in the chemical industry is limited due to its instability. When exposed to light, it can explode, and even diluted solutions are hazardous.
It is made by neutralizing chloric acid with ammonia or ammonium carbonate, or by precipitating barium, strontium, or calcium chlorates with ammonium carbonate or ammonium sulfate, yielding a carbonate or sulfate precipitate and an ammonium chlorate solution. Ammonium chlorate crystallizes as little needles that are easily dissolved in water.
Properties
Its molar mass is 101. 4897 g mol-1. It is a colorless crystal salt. Its density is 2.42 g mL-1 and its melting point is 380 ºC. However, in temperatures >142 ºC it decomposes to form nitrogen, chlorine and oxygen. It is soluble in water and diluted alcohol solutions but it is insoluble in pure alcohols.
- Chemical formula: NH4ClO3
- Appearance: small colorless crystals
- Density: 2.42 g/cm3
- Melting point: 380 °C (716 °F; 653 K) (decomposes)
- Magnetic susceptibility (χ): -42.1·10−6 cm3/mol
Preparation
Ammonium chlorate can be created by combining stoichiometric solutions of ammonium nitrate and sodium chlorate or ammonium sulfate and barium chlorate. It is created by neutralizing chloric acid with ammonia or ammonium carbonate:
HClO3 + NH3 → NH4ClO3
It can be prepared by reacting barium or calcium chlorate with ammonium sulfate to obtain the ammonium chlorate and barium or calcium sulfate. Calcium and barium cations form insoluble sulfates in water, thus it can be easily removed from the reaction mix.
Ca(ClO3)2 + (NH4)2SO4 → 2NH4ClO3 + CaSO4(s)
The bitartrate method is a viable production option if unusual chlorates are currently unavailable or must be manufactured. Warm potassium chlorate and ammonium bitartrate solutions are required. The latter can be made by combining aqueous ammonia with an excess of tartaric acid. The precipitation of ammonium chlorate will then occur as a result of a double displacement process.
Ammonium chlorate decomposes when heated to roughly 102 °C, releasing nitrogen, chlorine, and oxygen. It dissolves in diluted aqueous alcohol but not in strong alcohol. This compound is a strong oxidant and should never be stored with combustible materials because it can quickly generate explosive mixtures.
Occurrence
Because ammonium chlorate is so unstable, it has never been discovered in nature. It is a very unstable oxidizer that will disintegrate independently, sometimes violently, at ambient temperature. This is due to the interaction of the reducing ammonium cation and the oxidizing chlorate anion. Even solutions are known to be unstable. Because of the dangers of this salt, it should only be kept in solution when needed and should never be allowed to crystallize.
Uses
Ammonium chlorate has a fairly limited range of applications. The principal application for unstable salts is typically in the creation of explosive or chemical weapons.
Health effects/safety hazards
Ammonium chlorate is extremely hazardous. Its shipment is forbidden because to the significant risk of explosions. When exposed to light or heat, it can violently explode in concentrated solutions.