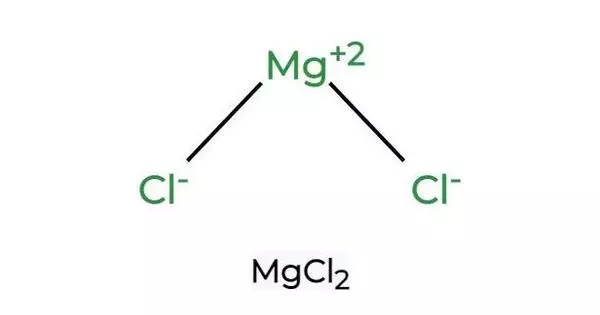
Magnesium chloride is an inorganic compound composed of one magnesium and two chloride ions. In medicine, the compound is used as a source of magnesium ions, which are essential for many cellular activities. This was used as a cathartic and in alloys.
Magnesium chloride is an inorganic compound family with the formula MgCl2•(H2O)x, where x can range from 0 to 12. These salts are colorless or white solids that are highly soluble in water. These naturally occurring compounds and their solutions have a wide range of applications. Anhydrous magnesium chloride is the primary precursor to magnesium metal, which is produced on a large scale. The most widely available form is hydrated magnesium chloride.
Properties
- Chemical formula: MgCl2
- Molar mass: 95.211 g/mol (anhydrous); 203.31 g/mol (hexahydrate)
- Appearance: white or colorless crystalline solid
- Density: 2.32 g/cm3 (anhydrous); 1.569 g/cm3 (hexahydrate)
- Melting point 714 °C (1,317 °F; 987 K) anhydrous
- Boiling point: 1,412 °C (2,574 °F; 1,685 K)
- Solubility in water: Anhydrous: 52.9 g/100 mL (0 °C); 72.6 g/100 mL (100 °C)
- Solubility: slightly soluble in acetone, pyridine
- Solubility in ethanol: 7.4 g/100 mL (30 °C)

Production
Brine or seawater can be used to extract magnesium chloride. It is primarily produced in North America from brine from the Great Salt Lake. It is obtained from the Dead Sea in the Jordan Valley. Bischofite (MgCl2•(H2O)6) is extracted (via solution mining) from ancient seabeds such as the Zechstein seabed in northwest Europe. Some deposits are the result of the primordial ocean’s high magnesium chloride content. Some magnesium chloride is produced through the evaporation of seawater.
The Dow process uses hydrochloric acid to regenerate magnesium chloride from magnesium hydroxide:
Mg(OH)2(s) + 2 HCl(aq) → MgCl2(aq) + 2 H2O(l)
It can also be prepared from magnesium carbonate by a similar reaction.
Occurrence
Magnesium concentrations in natural seawater range between 1250 and 1350 mg/L, accounting for approximately 3.7% of total seawater mineral content. The magnesium chloride ratio in Dead Sea minerals is significantly higher, at 50.8%. Carbonates and calcium are required for all coral, coralline algae, clams, and invertebrates to grow. Magnesium can be depleted by mangrove plants and excessive limewater use, as well as by exceeding natural calcium, alkalinity, and pH values.
Uses
- It is a precursor for magnesium metals.
- Used to control wind erosion, dust, and soil stabilization.
- Fire extinguishers use it.
- It is used as a food additive.
- Used in the production of paper.
- Used in the production of disinfectants.
- It is used as a flocculant and a catalyst.