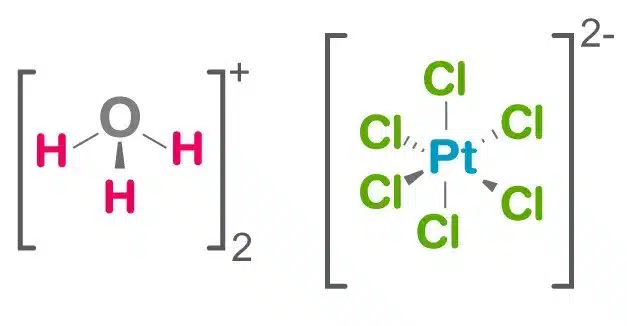
The inorganic compound chloroplatinic acid has the formula [H3O]2[PtCl6](H2O)x (0 ≤ x ≤ 6). It is a red solid that is a significant commercial source of platinum, usually in the form of an aqueous solution. It is the hydronium (H3O+) salt of the hexachloroplatinate anion (PtCl2-6), despite being abbreviated as H2PtCl6. Hexachloroplatinic acid is a highly hygroscopic substance.
Chloroplatinic acid is a red-orange powder or orange-red crystals. It is extremely soluble in water.
Properties
- Chemical formula: H6Cl6O2Pt
- Molar mass: 409.81 g/mol
- Appearance: Reddish brown solid
- Density: 2.431 g/cm3
- Melting point: 60 °C (140 °F; 333 K)
- Boiling point: decomposes
- Solubility in water: highly soluble
- Crystal structure: Anti-fluorite.
Production
Hexachloroplatinic acid can be made in a variety of ways. The most common of these methods is platinum dissolution in aqua regia. Other methods include electrolysis and exposing an aqueous suspension of platinum particles to chlorine gas.
Hexachloroplatinic acid is thought to be produced via the aqua regia route by the following equation:
Pt + 4 HNO3 + 6 HCl → H2PtCl6 + 4 NO2 + 4 H2O
The orange/red solution that results can be evaporated to produce brownish red crystals. Some authors believe that the hexachloroplatinic acid produced by this method contains nitrosonium hexachloroplatinate. According to newer literature, this is not the case, and that once the nitric acid has been removed, samples prepared using this method contain no detectable nitrogen.
Alternative methods have been investigated and described, with the goal of avoiding nitrogen contamination.
Reactions
When heated, hexachloroplatinic acid decomposes to platinum(IV) chloride.
(H3O)2PtCl6·nH2O → PtCl4 + 2 HCl + (n + 2) H2O
Synthesis
Chloroplatinic acid can be synthesized by dissolving platinum metal or platinum oxide in aqua regia (a mixture of concentrated nitric acid and hydrochloric acid). The resulting solution is evaporated to yield chloroplatinic acid.
Uses
Chloroplatinic acid is a common catalyst and precursor in the synthesis of various platinum compounds. It’s used to make pharmaceuticals, fragrances, dyes, and other organic compounds. It’s also used to make platinum-based catalysts for chemical reactions, fuel cells, and the oil industry.
Safety
Chloroplatinic acid is a corrosive and toxic substance. It can cause severe burns if swallowed, inhaled, or comes into contact with the skin. It should be handled with care and in a well-ventilated area.