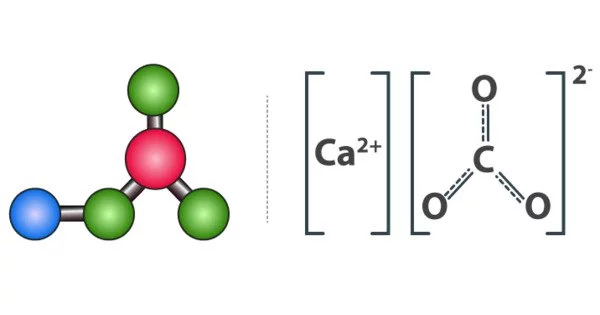
Calcium carbonate is a chemical compound with the formula CaCO. It is a common substance found in rocks as the minerals calcite and aragonite (most notably as limestone, a type of sedimentary rock composed primarily of calcite) and is the main component of eggshells, gastropod shells, shellfish skeletons, and pearls. Calcareous refers to substances that contain or resemble calcium carbonate.
Calcium carbonate is the active ingredient in agricultural lime and is formed when calcium ions in hard water react with carbonate ions to form limescale. It is used in medicine as a calcium supplement or as an antacid, but excessive consumption can be harmful, causing hypercalcemia and digestive issues.
Properties
Calcium carbonate appears as white, odorless powder or colorless crystals. It is practically insoluble in water. It occurs extensively in rocks worldwide. Ground calcium carbonate results directly from the mining of limestone.
- Chemical formula: CaCO3
- Molar mass: 100.0869 g/mol
- Appearance: Fine white powder; chalky taste
- Odor: odorless
- Density: 2.711 g/cm3 (calcite); 2.83 g/cm3 (aragonite)
- Melting point: 1,339°C (2,442 °F; 1,612 K) (calcite); 825 °C (1,517 °F; 1,098 K) (aragonite)
- Boiling point: decomposes
- Solubility in water: 0.013 g/L (25 °C)
- Solubility in dilute acids: soluble
- Crystal structure: Trigonal

Preparation
The vast majority of calcium carbonate used in industry is mined or quarried. Pure calcium carbonate (for food or pharmaceutical applications) can be produced from a pure quarried source (usually marble).
Calcium carbonate can also be made from calcium oxide. Water is added to produce calcium hydroxide, and then carbon dioxide is passed through the solution to precipitate the desired calcium carbonate, also known as precipitated calcium carbonate in the industry (PCC) This is known as carbonatation:
CaO + H2O → Ca(OH)2
Ca(OH)2 + CO2 → CaCO2 + H2O
Calcium carbonate can be easily crystallized from calcium chloride (CaCl2) in a laboratory by placing an aqueous solution of CaCl2 in a desiccator alongside ammonium carbonate (NH4)2CO3. Ammonium carbonate is exposed to air in the desiccator and decomposes into ammonia, carbon dioxide, and water. The carbon dioxide then diffuses into the calcium chloride aqueous solution, reacts with the calcium ions and water, and forms calcium carbonate.
Structure
The thermodynamically stable form of CaCO3 under normal conditions is hexagonal β-CaCO3 (the mineral calcite). Other forms can be prepared, the denser (2.83 g/cm3) orthorhombic λ-CaCO3 (the mineral aragonite) and hexagonal μ-CaCO3, occurring as the mineral vaterite. The aragonite form can be prepared by precipitation at temperatures above 85 °C; the vaterite form can be prepared by precipitation at 60 °C. Calcite contains calcium atoms coordinated by six oxygen atoms; in aragonite they are coordinated by nine oxygen atoms.
Occurrence
- Geological sources
Pure calcium carbonate minerals include calcite, aragonite, and vaterite. Limestone, chalk, marble, and travertine are examples of industrially important calcium carbonate source rocks.
- Biological sources
Eggshells, snail shells, and most seashells are high in calcium carbonate and can be used as industrial sources. Oyster shells have recently gained popularity as a dietary calcium source, but they are also a practical industrial source. Although dark green vegetables like broccoli and kale contain significant amounts of calcium carbonate, they are not suitable as an industrial source.
- Extraterrestrial
Beyond Earth, strong evidence suggests the presence of calcium carbonate on Mars. Signs of calcium carbonate have been detected at more than one location (notably at Gusev and Huygens craters). This provides some evidence for the past presence of liquid water.
Applications
Calcium carbonate is a dietary supplement that is used when the amount of calcium in the diet is insufficient. The body requires calcium to maintain healthy bones, muscles, the nervous system, and the heart. Calcium carbonate is also used as an antacid to treat heartburn, acid indigestion, and stomach upset.
Calcium carbonate is a dietary supplement that is used when the amount of calcium in the diet is insufficient. The body requires calcium to maintain healthy bones, muscles, the nervous system, and the heart. Calcium carbonate is also used as an antacid to treat heartburn, acid indigestion, and stomach upset.