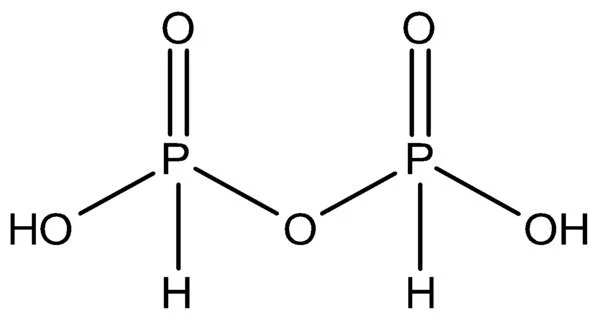
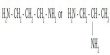Pyrophosphoric acid, also known as diphosphoric acid, is an inorganic compound with the formula H4P2O7 or, to put it another way, [(HO)2P(O)]2O. It has no color or odor and is soluble in water, diethyl ether, and ethyl alcohol. The anhydrous acid crystallizes into two polymorphs that melt at 54.3 and 71.5 °C, respectively.
Polyphosphoric acid, a major source of phosphoric acid, contains the compound. Pyrophosphates are anions, salts, and esters of pyrophosphoric acid. It has the chemical formula H4P2O7 and is an inorganic compound. It is created by dehydrating phosphoric acid (H3PO4). Pyrophosphoric acid is a white crystalline solid that is extremely water soluble.
Physical Properties:
- Pyrophosphoric acid is a white crystalline solid at room temperature.
- It has a molecular weight of approximately 177.99 g/mol.
- The compound has a density of about 2.02 g/cm³.
Chemical Properties:
- Pyrophosphoric acid is highly hygroscopic, meaning it readily absorbs moisture from the air.
- It is a strong acid and readily donates protons (H+) in aqueous solutions.
- The compound is an anhydride of phosphoric acid and can react with water to form phosphoric acid.
- It can undergo hydrolysis reactions, breaking down into phosphoric acid and water.
- It can form various salts, known as pyrophosphates, by reacting with metal cations.
Preparation
It can be prepared by reaction of phosphoric acid with phosphoryl chloride:
5 H3PO4 + POCl3 → 3 H4P2O7 + 3 HCl
Ion exchange from sodium pyrophosphate or hydrogen sulfide treatment of lead pyrophosphate can also be used to make it.
Boiling the water from orthophosphoric acid produces a mixture of ortho, pyro, and polyphosphoric acids, with the maximum pyrophosphoric acid concentration remaining below 50% and occurring slightly before what would otherwise be pure pyrophosphoric acid.
Pyrophosphoric acid is an intermediate compound in a variety of phosphate-related chemical reactions. It is frequently used in the synthesis of phosphate esters, which have applications in biochemistry, pharmaceuticals, and detergents. Pyrophosphoric acid can also function as a chelating agent, aiding in the stabilization of metal ions in solution.
Applications
One of the significant applications of pyrophosphoric acid is in the food industry. It is used as a food additive (E450) for its acidity-regulating properties. It can modify the pH of food products and act as a buffering agent, improving their stability and shelf life.
- Pyrophosphoric acid is commonly used as a catalyst in organic synthesis reactions.
- It is used in the production of various chemicals, such as phosphates, detergents, and fertilizers.
- The compound finds application in the food industry as an additive, primarily as an acidity regulator.
- It is employed in the preparation of pharmaceuticals and pharmaceutical intermediates.
- It is also utilized in the synthesis of specialty polymers and resins.
Safety
It is important to handle pyrophosphoric acid with care as it is a corrosive substance that can cause severe burns. Proper safety precautions should be followed when working with this compound, including the use of appropriate protective equipment such as gloves, goggles, and lab coats.