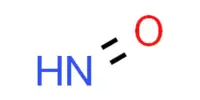
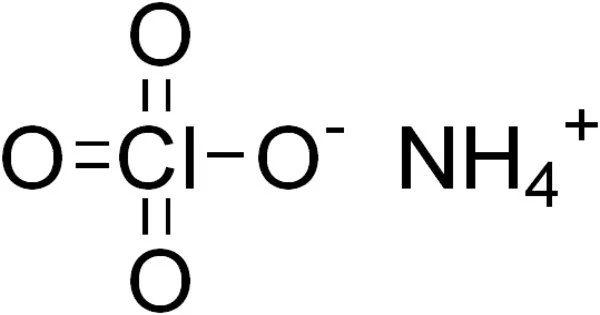The inorganic compound ammonium perchlorate (“AP”) has the formula NH4ClO4. It is a colorless or white solid that is water soluble. It is a strong oxidizer. It can be used as a rocket propellant when combined with a fuel. Because of its instability, it has been involved in a number of accidents, including the PEPCON disaster.
It is a significant oxidizer with a long history of use in solid rocket propellants, including space launch, military, amateur, and hobby high powered rockets, as well as some fireworks.
Properties
Ammonium perchlorate is a crystalline white solid or powder. It is a perchloric acid and ammonium hydroxide salt. All perchlorates have the potential to be strong oxidizers, but ammonium perchlorate is particularly labile. When ammonium perchlorate is combined with a fuel, it can produce self-sustaining combustion even at low atmospheric pressure.
- Chemical formula: NH4ClO4
- Molar mass: 117.49 g/mol
- Appearance: White Crystalline
- Density: 1.95 g/cm3
- Melting point: Exothermic decomposition before melting at >200 °C
- Solubility in water: 11.56 g/100 mL (0 °C); 57.01 g/100 mL (100 °C)
- Solubility: Soluble in Methanol; partially soluble in Acetone, Ethanol; insoluble in Ether
- Crystal structure: Orthorhombic (< 513 K); Cubic (> 513 K)

Production
Ammonium perchlorate (AP) is formed by the reaction of ammonia and perchloric acid. This is the primary process for the industrial production of perchloric acid. The salt can also be produced through the salt metathesis reaction of ammonium salts with sodium perchlorate. This method takes advantage of the relatively low solubility of NH4ClO4, which is about 10% that of sodium perchlorate.
It is a crystalline salt that is primarily used as an oxidizer in explosives, fireworks, and solid propellant systems for rockets. AP crystallizes as colorless rhombohedra.
Decomposition
Like most ammonium salts, ammonium perchlorate decomposes before melting. Mild heating results in production of hydrogen chloride, nitrogen, oxygen, and water.
4 NH4ClO4 → 4 HCl + 2 N2 + 5 O2 + 6 H2O
The combustion of AP is quite complex and has received a lot of attention. AP crystals decompose before melting, despite the presence of a thin liquid layer on crystal surfaces during high-pressure combustion processes. Strong heating can cause explosions. Complete reactions leave no trace of their existence. Pure crystals cannot sustain a flame at pressures lower than 2 MPa.
For particle sizes greater than 15 micrometres, AP is classified as a Class 4 oxidizer (can undergo an explosive reaction) and as an explosive for particle sizes less than 15 micrometres.
Applications
Ammonium perchlorate is primarily used in the production of solid fuel propellants. When AP is combined with a fuel (such as powdered aluminum and/or an elastomeric binder), it can produce self-sustaining combustion at pressures well below atmospheric pressure. It is a significant oxidizer with a long history of use in solid rocket propellants, including space launch (including the Space Shuttle Solid Rocket Booster), military, amateur, and hobby high-power rockets, and some fireworks.
Some “breakable” epoxy adhesives include AP suspensions. When heated to 300 °C, the AP degrades the organic adhesive, causing the cemented joint to break.
Toxicity
Perchlorate itself has low acute toxicity. Sodium perchlorate, for example, has an LD50 of 2-4 g/kg and is rapidly eliminated after ingestion. Chronic exposure to perchlorates, even at low concentrations, has been shown to cause a variety of thyroid problems because it is absorbed in place of iodine.