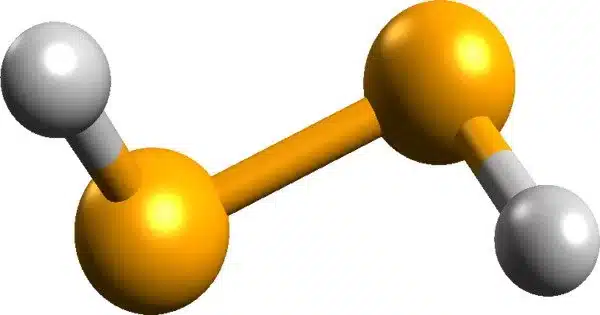
Hydrogen diselenide, also known as (SeH)2, is an inorganic selenium compound with the chemical formula H2Se2. It is a chemical compound made up of the elements hydrogen and selenium. It is a colorless, odorless gas that is highly toxic and corrosive. At room temperature, hydrogen diselenide easily dissociates to hydrogen selenide (H2Se) and elemental selenium, making it unstable. However, in some solutions, hydrogen diselenide can be stable.
Properties
It is a colorless, poisonous gas with a pungent odor, similar to hydrogen sulfide (H2S). It is highly reactive and can be considered an analog of hydrogen sulfide, but with a higher toxicity level.
- Chemical formula: H2Se2
- Appearance: oily liquid
- Flash point: Flammable
- State: It is a gas at room temperature and pressure.
- Odor: It has a characteristic pungent odor, resembling that of rotten eggs, similar to hydrogen sulfide.
- Color: It is a colorless gas.
Hydrogen diselenide is highly reactive and can readily react with various substances. It is a weak acid, capable of donating a hydrogen ion (H+) in solution. It is sparingly soluble in water, with solubility increasing with decreasing temperature.
Preparation
Hydrogen diselenide can be prepared by the reaction of hydrogen gas (H2) with elemental selenium (Se) at elevated temperatures. The reaction can be represented by the following equation:
H2 + Se → H2Se
The gas is denser than air and can form explosive mixtures when combined with air or oxygen. It is soluble in water, and the resulting solution is called selenic acid.
Hydrogen diselenide should be handled with extreme caution due to its toxic and corrosive properties. On contact, it can cause severe burns and eye damage, and it can be fatal if inhaled or ingested. When working with this compound, proper safety precautions should be taken, including the use of protective equipment and working in a well-ventilated area.
Applications
Hydrogen diselenide has few commercial applications due to its toxicity and hazardous nature. As a source of selenium, it may have limited utility in chemical synthesis or research.