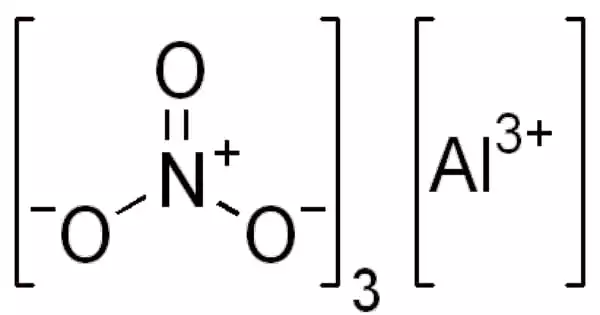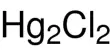Aluminium nitrate is a white, water-soluble compound of aluminum and nitric acid that is most usually found as the crystalline hydrate, Al(NO3)3•9H2O. It is a water-soluble salt of nitric acid and aluminum. It has a white color to it. This substance can be found as a crystalline solid or as a powder. Aluminum nitrate is normally solid at standard temperature and pressure conditions.
This chemical appears to be extremely lucrative and has a variety of essential applications. To begin with, aluminum nitrate appears to be a powerful oxidizing agent. As a result, it is absolutely important during the oxidization process.
Properties
Aluminum nitrate is a crystalline white substance. It is a chemical that appears to be a nitric acid and aluminum salt. Aluminum nitrate exists as a crystalline hydrate under normal circumstances. Aluminum nitrate’s visual shape is usually crystalline solid or powder with a light color.
- Chemical formula: Al(NO3)3
- Molar mass: 212.996 g/mol (anhydrous); 375.134 g/mol (nonahydrate)
- Appearance: White crystals, solid hygroscopic
- Odor: odorless
- Density: 1.72 g/cm3 (nonahydrate)
- Melting point: 66 °C (151 °F) (anhydrous); 73.9 °C (165.0 °F) (nonahydrate)
- Boiling point: 150 °C (302 °F) (nonahydrate) decomposes
- Solubility in methanol: 14.45 g/100ml
- Solubility in ethanol: 8.63 g/100ml
- Solubility in ethylene glycol: 18.32 g/100ml
This chemical has a wide range of physical properties. Aluminum nitrate, for example, has a molar mass of 212.996 g/mol. The compound has a density of 1.401 g/cm3 and a volume of 1.401 g/cm3. Furthermore, aluminum nitrate, like any other compound, has melting and boiling temperatures of 72.8°C and 135°C, respectively. Aluminum nitrate is reported to be soluble in a variety of liquids. Aluminum nitrate has no odor and appears to have a molecular weight of 375.13.

Preparation
Aluminium nitrate cannot be synthesized by the reaction of aluminium with concentrated nitric acid, as the aluminium forms a passivation layer.
Aluminium nitrate may instead be prepared by the reaction of nitric acid with aluminium(III) chloride. Nitrosyl chloride is produced as a by-product; it bubbles out of the solution as a gas. More conveniently, the salt can be made by reacting nitric acid with aluminium hydroxide.
Aluminium nitrate may also be prepared a metathesis reaction between aluminium sulfate and a nitrate salt with a suitable cation such as barium, strontium, calcium, silver, or lead. e.g. Al2(SO4)3 + 3 Ba(NO3)2 → 2 Al(NO3)3 + 3 BaSO4.
Uses
Aluminium nitrate is a powerful oxidizer. It is used in leather tanning, antiperspirants, corrosion inhibitors, uranium extraction, petroleum refining, and as a nitrating agent.
Aluminum nitrate is used in uranium extraction. The extraction of uranium from the ground is the process of uranium mining. Given the importance of uranium, it is difficult to envision modern life without it. Aluminum nitrate is employed in this uranium extraction procedure. Without aluminum nitrate, carrying out the extraction would be much more difficult.
There are numerous applications for nonahydrate and other hydrated aluminum nitrates. These salts are used to make alumina, which is then used to make insulating sheets, cathode ray tube heating elements, and transformer core laminates. Actinide elements are extracted using hydrated salts as well.
It is used in the laboratory and classroom such as in the reaction
Al(NO3)3 + 3 NaOH → Al(OH)3 + 3 NaNO3
It is, however, much less often encountered than aluminium chloride and aluminium sulfate.
Safety
Noncombustible, yet can hasten the combustion of flammable elements. An explosion may occur if significant volumes are involved or the combustible substance is finely split. An explosion may occur if exposed to fire or heat for an extended period of time. Nitrogen oxides are produced in fires involving this substance. Petroleum refining, dyeing, and leather tanning are just a few of the applications.